PemK toxin of Bacillus anthracis is a ribonuclease: an insight into its active site, structure, and function
- PMID: 20022964
- PMCID: PMC2844174
- DOI: 10.1074/jbc.M109.073387
PemK toxin of Bacillus anthracis is a ribonuclease: an insight into its active site, structure, and function
Abstract
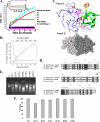
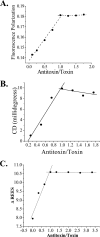
Bacillus anthracis genome harbors a toxin-antitoxin (TA) module encoding pemI (antitoxin) and pemK (toxin). This study describes the rPemK as a potent ribonuclease with a preference for pyrimidines (C/U), which is consistent with our previous study that demonstrated it as a translational attenuator. The in silico structural modeling of the PemK in conjunction with the site-directed mutagenesis confirmed the role of His-59 and Glu-78 as an acid-base couple in mediating the ribonuclease activity. The rPemK is shown to form a complex with the rPemI, which is in line with its function as a TA module. This rPemI-rPemK complex becomes catalytically inactive when both the proteins interact in a molar stoichiometry of 1. The rPemI displays vulnerability to proteolysis but attains conformational stability only upon rPemK interaction. The pemI-pemK transcript is shown to be up-regulated upon stress induction with a concomitant increase in the amount of PemK and a decline in the PemI levels, establishing the role of these modules in stress. The artificial perturbation of TA interaction could unleash the toxin, executing bacterial cell death. Toward this end, synthetic peptides are designed to disrupt the TA interaction. The peptides are shown to be effective in abrogating TA interaction in micromolar range in vitro. This approach can be harnessed as a potential antibacterial strategy against anthrax in the future.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

