Activation of nerve growth factor-induced B alpha by methylene-substituted diindolylmethanes in bladder cancer cells induces apoptosis and inhibits tumor growth
- PMID: 20023005
- PMCID: PMC2835419
- DOI: 10.1124/mol.109.061143
Activation of nerve growth factor-induced B alpha by methylene-substituted diindolylmethanes in bladder cancer cells induces apoptosis and inhibits tumor growth
Abstract
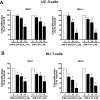
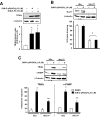
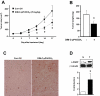
Nerve growth factor-induced B (NGFI-B) genes are orphan nuclear receptors, and NGFI-B alpha (Nur77, TR3) is overexpressed in bladder tumors and bladder cancer cells compared with nontumorous bladder tissue. 1,1-Bis(3'-indolyl)-1-(p-methoxyphenyl)-methane (DIM-C-pPhOCH(3)) and 1,1-bis(3'-indolyl)-1-(p-phenyl)methane have previously been identified as activators of Nur77, and both compounds inhibited growth and induced apoptosis of UC-5 and KU7 bladder cancer cells. The proapoptotic effects of methylene-substituted diindolylmethanes (C-DIMs) were unaffected by cotreatment with leptomycin B and were dependent on nuclear Nur77, and RNA interference with a small inhibitory RNA for Nur77 (iNur77) demonstrated that C-DIM-induced activation of apoptosis was Nur77-dependent. Microarray analysis of DIM-C-pPhOCH(3)-induced genes in UC-5 bladder cancer cells showed that this compound induced multiple Nur77-dependent proapoptotic or growth inhibitory genes including tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), cystathionase, p21, p8, and sestrin-2. DIM-C-pPhOCH(3) (25 mg/kg/d) also induced apoptosis and inhibited tumor growth in athymic nude mice bearing KU7 cells as xenografts, demonstrating that Nur77-active C-DIMs exhibit potential for bladder cancer chemotherapy by targeting Nur77, which is overexpressed in this tumor type.
Figures



Similar articles
-
Targeting NR4A1 (TR3) in cancer cells and tumors.Expert Opin Ther Targets. 2011 Feb;15(2):195-206. doi: 10.1517/14728222.2011.547481. Epub 2011 Jan 5. Expert Opin Ther Targets. 2011. PMID: 21204731 Free PMC article. Review.
-
Activation of nuclear TR3 (NR4A1) by a diindolylmethane analog induces apoptosis and proapoptotic genes in pancreatic cancer cells and tumors.Carcinogenesis. 2011 Jun;32(6):836-42. doi: 10.1093/carcin/bgr040. Epub 2011 Mar 1. Carcinogenesis. 2011. PMID: 21362629 Free PMC article.
-
1,1-bis(3'-indolyl)-1-(p-methoxyphenyl)methane activates Nur77-independent proapoptotic responses in colon cancer cells.Mol Carcinog. 2008 Apr;47(4):252-63. doi: 10.1002/mc.20378. Mol Carcinog. 2008. PMID: 17957723
-
Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and nuclear receptor-independent pathways.Cancer Res. 2007 Jan 15;67(2):674-83. doi: 10.1158/0008-5472.CAN-06-2907. Cancer Res. 2007. PMID: 17234778
-
Cancer chemotherapy with indole-3-carbinol, bis(3'-indolyl)methane and synthetic analogs.Cancer Lett. 2008 Oct 8;269(2):326-38. doi: 10.1016/j.canlet.2008.04.021. Epub 2008 May 22. Cancer Lett. 2008. PMID: 18501502 Free PMC article. Review.
Cited by
-
Regulation of Nur77 expression by β-catenin and its mitogenic effect in colon cancer cells.FASEB J. 2011 Jan;25(1):192-205. doi: 10.1096/fj.10-166462. Epub 2010 Sep 16. FASEB J. 2011. PMID: 20847229 Free PMC article.
-
Diindolylmethane analogs bind NR4A1 and are NR4A1 antagonists in colon cancer cells.Mol Endocrinol. 2014 Oct;28(10):1729-39. doi: 10.1210/me.2014-1102. Epub 2014 Aug 6. Mol Endocrinol. 2014. PMID: 25099012 Free PMC article.
-
Compensatory Expression of Nur77 and Nurr1 Regulates NF-κB-Dependent Inflammatory Signaling in Astrocytes.Mol Pharmacol. 2018 Oct;94(4):1174-1186. doi: 10.1124/mol.118.112631. Epub 2018 Aug 15. Mol Pharmacol. 2018. PMID: 30111648 Free PMC article.
-
Targeting NR4A1 (TR3) in cancer cells and tumors.Expert Opin Ther Targets. 2011 Feb;15(2):195-206. doi: 10.1517/14728222.2011.547481. Epub 2011 Jan 5. Expert Opin Ther Targets. 2011. PMID: 21204731 Free PMC article. Review.
-
Activation of nuclear TR3 (NR4A1) by a diindolylmethane analog induces apoptosis and proapoptotic genes in pancreatic cancer cells and tumors.Carcinogenesis. 2011 Jun;32(6):836-42. doi: 10.1093/carcin/bgr040. Epub 2011 Mar 1. Carcinogenesis. 2011. PMID: 21362629 Free PMC article.
References
-
- Abdelrahim M, Newman K, Vanderlaag K, Samudio I, Safe S. (2006) 3,3′-Diindolylmethane (DIM) and derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis 27:717–728 - PubMed
-
- Abdelrahim M, Samudio I, Smith R, 3rd, Burghardt R, Safe S. (2002) Small inhibitory RNA duplexes for Sp1 mRNA block basal and estrogen-induced gene expression and cell cycle progression in MCF-7 breast cancer cells. J Biol Chem 277:28815–28822 - PubMed
-
- Baek SJ, Kim JS, Jackson FR, Eling TE, McEntee MF, Lee SH. (2004a) Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis 25:2425–2432 - PubMed
-
- Baek SJ, Kim JS, Nixon JB, DiAugustine RP, Eling TE. (2004b) Expression of NAG-1, a transforming growth factor-β superfamily member, by troglitazone requires the early growth response gene EGR-1. J Biol Chem 279:6883–6892 - PubMed
-
- Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. (2001) Cyclooxygenase inhibitors regulate the expression of a TGF-β superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol 59:901–908 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

