CotE binds to CotC and CotU and mediates their interaction during spore coat formation in Bacillus subtilis
- PMID: 20023017
- PMCID: PMC2812956
- DOI: 10.1128/JB.01408-09
CotE binds to CotC and CotU and mediates their interaction during spore coat formation in Bacillus subtilis
Abstract
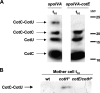
CotE is a morphogenic protein that controls the assembly of the coat, the proteinaceous structure that surrounds and protects the spore of Bacillus subtilis. CotE has long been thought to interact with several outer coat components, but such interactions were hypothesized from genetic experiment results and have never been directly demonstrated. To study the interaction of CotE with other coat components, we focused our attention on CotC and CotU, two outer coat proteins known to be under CotE control and to form a heterodimer. We report here the results of pull-down experiments that provide the first direct evidence that CotE contacts other coat components. In addition, coexpression experiments demonstrate that CotE is needed and sufficient to allow formation of the CotC-CotU heterodimer in a heterologous host.
Figures


Similar articles
-
CotC-CotU heterodimerization during assembly of the Bacillus subtilis spore coat.J Bacteriol. 2008 Feb;190(4):1267-75. doi: 10.1128/JB.01425-07. Epub 2007 Dec 7. J Bacteriol. 2008. PMID: 18065538 Free PMC article.
-
Functional regions of the Bacillus subtilis spore coat morphogenetic protein CotE.J Bacteriol. 1999 Nov;181(22):7043-51. doi: 10.1128/JB.181.22.7043-7051.1999. J Bacteriol. 1999. PMID: 10559171 Free PMC article.
-
Gene encoding a morphogenic protein required in the assembly of the outer coat of the Bacillus subtilis endospore.Genes Dev. 1988 Aug;2(8):1047-54. doi: 10.1101/gad.2.8.1047. Genes Dev. 1988. PMID: 3139490
-
[Overview of study on Bacillus subtilis spores].Yakugaku Zasshi. 2013;133(7):783-97. doi: 10.1248/yakushi.13-00143. Yakugaku Zasshi. 2013. PMID: 23811766 Review. Japanese.
-
Maximum shields: the assembly and function of the bacterial spore coat.Trends Microbiol. 2002 Jun;10(6):251-4. doi: 10.1016/s0966-842x(02)02373-9. Trends Microbiol. 2002. PMID: 12088650 Review.
Cited by
-
Organization and evolution of the cotG and cotH genes of Bacillus subtilis.J Bacteriol. 2011 Dec;193(23):6664-73. doi: 10.1128/JB.06121-11. Epub 2011 Oct 7. J Bacteriol. 2011. PMID: 21984783 Free PMC article.
-
The Direct Interaction between Two Morphogenetic Proteins Is Essential for Spore Coat Formation in Bacillus subtilis.PLoS One. 2015 Oct 20;10(10):e0141040. doi: 10.1371/journal.pone.0141040. eCollection 2015. PLoS One. 2015. PMID: 26484546 Free PMC article.
-
Progress in research and application development of surface display technology using Bacillus subtilis spores.Appl Microbiol Biotechnol. 2020 Mar;104(6):2319-2331. doi: 10.1007/s00253-020-10348-x. Epub 2020 Jan 27. Appl Microbiol Biotechnol. 2020. PMID: 31989224 Free PMC article. Review.
-
Antagonistic role of CotG and CotH on spore germination and coat formation in Bacillus subtilis.PLoS One. 2014 Aug 12;9(8):e104900. doi: 10.1371/journal.pone.0104900. eCollection 2014. PLoS One. 2014. PMID: 25115591 Free PMC article.
-
Bacterial Spore-Based Delivery System: 20 Years of a Versatile Approach for Innovative Vaccines.Biomolecules. 2023 Jun 6;13(6):947. doi: 10.3390/biom13060947. Biomolecules. 2023. PMID: 37371527 Free PMC article. Review.
References
-
- Baccigalupi, L., G. Castaldo, G. Cangiano, R. Isticato, R. Marasco, M. De Felice, and E. Ricca. 2004. GerE-independent expression of cotH leads to CotC accumulation in the mother cell compartment during Bacillus subtilis sporulation. Microbiology 150:3441-3449. - PubMed
-
- Chambery, A., A. Farina, A. Di Maro, M. Rossi, C. Abbondanza, B. Moncharmont, L. Malorni, G. Cacace, G. Pocsfalvi, A. Malorni, and A. Parente. 2006. Proteomic analysis of MCF-7 cell lines expressing the zinc-finger or the proline-rich domain of retinoblastoma-interacting-zinc-finger protein. J. Proteome Res. 5:1176-1185. - PubMed
-
- Cutting, S., and P. B. Vander Horn. 1990. Genetic analysis, p. 27-74. In C. Harwood and S. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom.
-
- Driks, A. 2002. Maximum shields: the assembly and function of the bacterial spore coat. Trends Microbiol. 10:251-254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

