Bacterial ammeline metabolism via guanine deaminase
- PMID: 20023034
- PMCID: PMC2812975
- DOI: 10.1128/JB.01243-09
Bacterial ammeline metabolism via guanine deaminase
Abstract
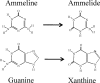
Melamine toxicity in mammals has been attributed to the blockage of kidney tubules by insoluble complexes of melamine with cyanuric acid or uric acid. Bacteria metabolize melamine via three consecutive deamination reactions to generate cyanuric acid. The second deamination reaction, in which ammeline is the substrate, is common to many bacteria, but the genes and enzymes responsible have not been previously identified. Here, we combined bioinformatics and experimental data to identify guanine deaminase as the enzyme responsible for this biotransformation. The ammeline degradation phenotype was demonstrated in wild-type Escherichia coli and Pseudomonas strains, including E. coli K12 and Pseudomonas putida KT2440. Bioinformatics analysis of these and other genomes led to the hypothesis that the ammeline deaminating enzyme was guanine deaminase. An E. coli guanine deaminase deletion mutant was deficient in ammeline deaminase activity, supporting the role of guanine deaminase in this reaction. Two guanine deaminases from disparate sources (Bradyrhizobium japonicum USDA 110 and Homo sapiens) that had available X-ray structures were purified to homogeneity and shown to catalyze ammeline deamination at rates sufficient to support bacterial growth on ammeline as a sole nitrogen source. In silico models of guanine deaminase active sites showed that ammeline could bind to guanine deaminase in a similar orientation to guanine, with a favorable docking score. Other members of the amidohydrolase superfamily that are not guanine deaminases were assayed in vitro, and none had substantial ammeline deaminase activity. The present study indicated that widespread guanine deaminases have a promiscuous activity allowing them to catalyze a key reaction in the bacterial transformation of melamine to cyanuric acid and potentially contribute to the toxicity of melamine.
Figures
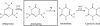



Similar articles
-
Melamine deaminase and atrazine chlorohydrolase: 98 percent identical but functionally different.J Bacteriol. 2001 Apr;183(8):2405-10. doi: 10.1128/JB.183.8.2405-2410.2001. J Bacteriol. 2001. PMID: 11274097 Free PMC article.
-
Structural Determinants for Substrate Selectivity in Guanine Deaminase Enzymes of the Amidohydrolase Superfamily.Biochemistry. 2019 Jul 30;58(30):3280-3292. doi: 10.1021/acs.biochem.9b00341. Epub 2019 Jul 19. Biochemistry. 2019. PMID: 31283204 Free PMC article.
-
Structural basis of the substrate specificity of cytidine deaminase superfamily Guanine deaminase.Biochemistry. 2013 Nov 12;52(45):8106-14. doi: 10.1021/bi400818e. Epub 2013 Oct 28. Biochemistry. 2013. PMID: 24083949
-
Bacterial catabolism of s-triazine herbicides: biochemistry, evolution and application.Adv Microb Physiol. 2020;76:129-186. doi: 10.1016/bs.ampbs.2020.01.004. Epub 2020 Feb 11. Adv Microb Physiol. 2020. PMID: 32408946 Review.
-
Recent advances in the risk assessment of melamine and cyanuric acid in animal feed.Toxicol Appl Pharmacol. 2013 Aug 1;270(3):218-29. doi: 10.1016/j.taap.2012.01.012. Epub 2012 Jan 25. Toxicol Appl Pharmacol. 2013. PMID: 22306862 Review.
Cited by
-
Depletion of melamine and cyanuric acid in serum from catfish Ictalurus punctatus and rainbow trout Onchorhynchus mykiss.Food Chem Toxicol. 2012 Oct;50(10):3426-32. doi: 10.1016/j.fct.2012.07.055. Epub 2012 Aug 4. Food Chem Toxicol. 2012. PMID: 22889901 Free PMC article. Clinical Trial.
-
Nucleobase deaminases: a potential enzyme system for new therapies.RSC Adv. 2018 Jun 28;8(42):23567-23577. doi: 10.1039/c8ra04112a. eCollection 2018 Jun 27. RSC Adv. 2018. PMID: 35540270 Free PMC article. Review.
-
A No Observable Adverse Effects Level (NOAEL) for pigs fed melamine and cyanuric acid.Regul Toxicol Pharmacol. 2011 Aug;60(3):363-72. doi: 10.1016/j.yrtph.2011.05.004. Epub 2011 May 18. Regul Toxicol Pharmacol. 2011. PMID: 21620919 Free PMC article.
-
Guanine crystal formation by bacteria.BMC Biol. 2023 Apr 3;21(1):66. doi: 10.1186/s12915-023-01572-8. BMC Biol. 2023. PMID: 37013555 Free PMC article.
-
Discovery of Hepatotoxic Equivalent Markers and Mechanism of Polygonum multiflorum Thunb. by Metabolomics Coupled with Molecular Docking.Molecules. 2022 Dec 21;28(1):25. doi: 10.3390/molecules28010025. Molecules. 2022. PMID: 36615221 Free PMC article.
References
-
- Brown, C. A., K. S. Jeong, R. H. Poppenga, B. Puschner, D. M. Miller, A. E. Ellis, K. Kang, S. Sum, A. M. Cistola, and S. A. Brown. 2007. Outbreaks of renal failure associated with melamine and cyanuric acid in dogs and cats in 2004 and 2007. J. Vet. Diagn. Invest. 19:525-531. - PubMed
-
- Dobson, R., S. Motlagh, M. Quijano, T. Cambron, A. Pullen, B. Regg, A. Gigalow-Kern, T. Vennard, A. Fix, R. Reimschuessel, G. Overmann, Y. Shan, and G. Daston. 2008. Identification and characterization of toxicity of contaminants in pet food leading to an outbreak of renal toxicity in cats and dogs. Toxicol. Sci. 106:251-262. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

