Metabolic differentiation in biofilms as indicated by carbon dioxide production rates
- PMID: 20023078
- PMCID: PMC2820951
- DOI: 10.1128/AEM.01719-09
Metabolic differentiation in biofilms as indicated by carbon dioxide production rates
Abstract
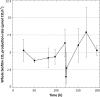
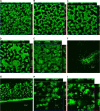
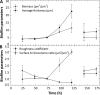
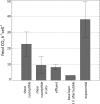
The measurement of carbon dioxide production rates as an indication of metabolic activity was applied to study biofilm development and response of Pseudomonas sp. biofilms to an environmental disturbance in the form of a moving air-liquid interface (i.e., shear). A differential response in biofilm cohesiveness was observed after bubble perturbation, and the biofilm layers were operationally defined as either shear-susceptible or non-shear-susceptible. Confocal laser scanning microscopy and image analysis showed a significant reduction in biofilm thickness and biomass after the removal of the shear-susceptible biofilm layer, as well as notable changes in the roughness coefficient and surface-to-biovolume ratio. These changes were accompanied by a 72% reduction of whole-biofilm CO2 production; however, the non-shear-susceptible region of the biofilm responded rapidly after the removal of the overlying cells and extracellular polymeric substances (EPS) along with the associated changes in nutrient and O2 flux, with CO2 production rates returning to preperturbation levels within 24 h. The adaptable nature and the ability of bacteria to respond to environmental conditions were further demonstrated by the outer shear-susceptible region of the biofilm; the average CO2 production rate of cells from this region increased within 0.25 h from 9.45 +/- 5.40 fmol of CO2 x cell(-1) x h(-1) to 22.6 +/- 7.58 fmol of CO2 x cell(-1) x h(-1) when cells were removed from the biofilm and maintained in suspension without an additional nutrient supply. These results also demonstrate the need for sufficient monitoring of biofilm recovery at the solid substratum if mechanical methods are used for biofouling control.
Figures


References
-
- Bester, E., E. Edwards, and G. M. Wolfaardt. 2009. Planktonic cell yield is linked to biofilm development. Can. J. Microbiol. 55:1195-1206. - PubMed
-
- Blanch, H. W. 1996. Biochemical engineering. M. Dekker, New York, NY.
-
- Bloemen, H. H. J., L. Wu, W. M. Van Gulik, J. J. Heijnen, and M. H. G. Verhaegen. 2003. Reconstruction of the O2 uptake rate and CO2 evolution rate on a time scale of seconds. AICHE J. 49:1895-1908.
-
- Bonarius, H. P. J., C. D. De Gooijer, J. Tramper, and G. Schmid. 1995. Determination of the respiration quotient in mammalian cell culture in bicarbonate buffered media. Biotechnol. Bioeng. 45:524-535. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

