Regulation of arabinose and xylose metabolism in Escherichia coli
- PMID: 20023096
- PMCID: PMC2832368
- DOI: 10.1128/AEM.01970-09
Regulation of arabinose and xylose metabolism in Escherichia coli
Abstract
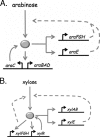
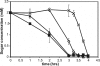
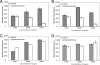
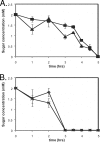
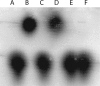
Bacteria such as Escherichia coli will often consume one sugar at a time when fed multiple sugars, in a process known as carbon catabolite repression. The classic example involves glucose and lactose, where E. coli will first consume glucose, and only when it has consumed all of the glucose will it begin to consume lactose. In addition to that of lactose, glucose also represses the consumption of many other sugars, including arabinose and xylose. In this work, we characterized a second hierarchy in E. coli, that between arabinose and xylose. We show that, when grown in a mixture of the two pentoses, E. coli will consume arabinose before it consumes xylose. Consistent with a mechanism involving catabolite repression, the expression of the xylose metabolic genes is repressed in the presence of arabinose. We found that this repression is AraC dependent and involves a mechanism where arabinose-bound AraC binds to the xylose promoters and represses gene expression. Collectively, these results demonstrate that sugar utilization in E. coli involves multiple layers of regulation, where cells will consume first glucose, then arabinose, and finally xylose. These results may be pertinent in the metabolic engineering of E. coli strains capable of producing chemical and biofuels from mixtures of hexose and pentose sugars derived from plant biomass.
Figures




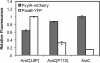

Similar articles
-
Regulation of metabolism in Escherichia coli during growth on mixtures of the non-glucose sugars: arabinose, lactose, and xylose.Sci Rep. 2018 Jan 12;8(1):609. doi: 10.1038/s41598-017-18704-0. Sci Rep. 2018. PMID: 29330542 Free PMC article.
-
Reciprocal Regulation of l-Arabinose and d-Xylose Metabolism in Escherichia coli.J Bacteriol. 2015 Nov 2;198(3):386-93. doi: 10.1128/JB.00709-15. Print 2016 Feb 1. J Bacteriol. 2015. PMID: 26527647 Free PMC article.
-
Simultaneous uptake of lignocellulose-based monosaccharides by Escherichia coli.Biotechnol Bioeng. 2014 Jun;111(6):1108-15. doi: 10.1002/bit.25182. Epub 2014 Jan 23. Biotechnol Bioeng. 2014. PMID: 24382675
-
Pentose metabolism and conversion to biofuels and high-value chemicals in yeasts.FEMS Microbiol Rev. 2021 Aug 17;45(4):fuaa069. doi: 10.1093/femsre/fuaa069. FEMS Microbiol Rev. 2021. PMID: 33316044 Review.
-
Ethanol production from lignocellulosic biomass by recombinant Escherichia coli strain FBR5.Bioengineered. 2012 Jul-Aug;3(4):197-202. doi: 10.4161/bioe.19874. Epub 2012 Jun 18. Bioengineered. 2012. PMID: 22705843 Free PMC article. Review.
Cited by
-
Displaced by Deceivers: Prevention of Biosensor Cross-Talk Is Pivotal for Successful Biosensor-Based High-Throughput Screening Campaigns.ACS Synth Biol. 2019 Aug 16;8(8):1847-1857. doi: 10.1021/acssynbio.9b00149. Epub 2019 Jul 23. ACS Synth Biol. 2019. PMID: 31268296 Free PMC article.
-
Transcriptional Control of Dual Transporters Involved in α-Ketoglutarate Utilization Reveals Their Distinct Roles in Uropathogenic Escherichia coli.Front Microbiol. 2017 Feb 21;8:275. doi: 10.3389/fmicb.2017.00275. eCollection 2017. Front Microbiol. 2017. PMID: 28270808 Free PMC article.
-
Aromatic inhibitors derived from ammonia-pretreated lignocellulose hinder bacterial ethanologenesis by activating regulatory circuits controlling inhibitor efflux and detoxification.Front Microbiol. 2014 Aug 13;5:402. doi: 10.3389/fmicb.2014.00402. eCollection 2014. Front Microbiol. 2014. PMID: 25177315 Free PMC article.
-
Experimental evolution reveals an effective avenue to release catabolite repression via mutations in XylR.Proc Natl Acad Sci U S A. 2017 Jul 11;114(28):7349-7354. doi: 10.1073/pnas.1700345114. Epub 2017 Jun 27. Proc Natl Acad Sci U S A. 2017. PMID: 28655843 Free PMC article.
-
Arabinose-Induced Catabolite Repression as a Mechanism for Pentose Hierarchy Control in Clostridium acetobutylicum ATCC 824.mSystems. 2018 Oct 23;3(5):e00064-18. doi: 10.1128/mSystems.00064-18. eCollection 2018 Sep-Oct. mSystems. 2018. PMID: 30374459 Free PMC article.
References
-
- Aristidou, A., and M. Penttila. 2000. Metabolic engineering applications to renewable resource utilization. Curr. Opin. Biotechnol. 11:187-198. - PubMed
-
- Bailey, T. L., and C. Elkan. 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers, p. 28-36. In R. Altman, D. Brutlag, P. Karp, R. Lathrop, and D. Searls (ed.), Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology. AAAI Press, Menlo Park, CA. - PubMed
-
- Bothast, R. J., B. C. Saha, A. V. Flosenzier, and L. O. Ingram. 1994. Fermentation of l-arabinose, d-xylose and d-glucose by ethanologenic recombinant Klebsiella oxytoca strain P2. Biotechnol. Lett. 16:401-406.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

