Yeast surface display of trifunctional minicellulosomes for simultaneous saccharification and fermentation of cellulose to ethanol
- PMID: 20023102
- PMCID: PMC2820948
- DOI: 10.1128/AEM.01687-09
Yeast surface display of trifunctional minicellulosomes for simultaneous saccharification and fermentation of cellulose to ethanol
Abstract
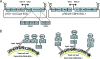
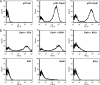
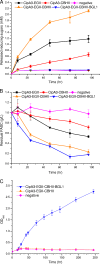
By combining cellulase production, cellulose hydrolysis, and sugar fermentation into a single step, consolidated bioprocessing (CBP) represents a promising technology for biofuel production. Here we report engineering of Saccharomyces cerevisiae strains displaying a series of uni-, bi-, and trifunctional minicellulosomes. These minicellulosomes consist of (i) a miniscaffoldin containing a cellulose-binding domain and three cohesin modules, which was tethered to the cell surface through the yeast a-agglutinin adhesion receptor, and (ii) up to three types of cellulases, an endoglucanase, a cellobiohydrolase, and a beta-glucosidase, each bearing a C-terminal dockerin. Cell surface assembly of the minicellulosomes was dependent on expression of the miniscaffoldin, indicating that formation of the complex was dictated by the high-affinity interactions between cohesins and dockerins. Compared to the unifunctional and bifunctional minicellulosomes, the quaternary trifunctional complexes showed enhanced enzyme-enzyme synergy and enzyme proximity synergy. More importantly, surface display of the trifunctional minicellulosomes gave yeast cells the ability to simultaneously break down and ferment phosphoric acid-swollen cellulose to ethanol with a titer of approximately 1.8 g/liter. To our knowledge, this is the first report of a recombinant yeast strain capable of producing cell-associated trifunctional minicellulosomes. The strain reported here represents a useful engineering platform for developing CBP-enabling microorganisms and elucidating principles of cellulosome construction and mode of action.
Figures


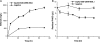
Similar articles
-
Self-surface assembly of cellulosomes with two miniscaffoldins on Saccharomyces cerevisiae for cellulosic ethanol production.Proc Natl Acad Sci U S A. 2012 Aug 14;109(33):13260-5. doi: 10.1073/pnas.1209856109. Epub 2012 Aug 1. Proc Natl Acad Sci U S A. 2012. PMID: 22853950 Free PMC article.
-
Production of minicellulosomes from Clostridium cellulovorans for the fermentation of cellulosic ethanol using engineered recombinant Saccharomyces cerevisiae.FEMS Microbiol Lett. 2010 Sep 1;310(1):39-47. doi: 10.1111/j.1574-6968.2010.02035.x. Epub 2010 Jun 16. FEMS Microbiol Lett. 2010. PMID: 20637040
-
Engineered pentafunctional minicellulosome for simultaneous saccharification and ethanol fermentation in Saccharomyces cerevisiae.Appl Environ Microbiol. 2014 Nov;80(21):6677-84. doi: 10.1128/AEM.02070-14. Epub 2014 Aug 22. Appl Environ Microbiol. 2014. PMID: 25149522 Free PMC article.
-
[Progress and strategies on bioethanol production from lignocellulose by consolidated bioprocessing (CBP) using Saccharomyces cerevisiae].Sheng Wu Gong Cheng Xue Bao. 2010 Jul;26(7):870-9. Sheng Wu Gong Cheng Xue Bao. 2010. PMID: 20954386 Review. Chinese.
-
Engineering microbes for direct fermentation of cellulose to bioethanol.Crit Rev Biotechnol. 2018 Nov;38(7):1089-1105. doi: 10.1080/07388551.2018.1452891. Epub 2018 Apr 10. Crit Rev Biotechnol. 2018. PMID: 29631429 Review.
Cited by
-
Assembly of minicellulosomes on the surface of Bacillus subtilis.Appl Environ Microbiol. 2011 Jul;77(14):4849-58. doi: 10.1128/AEM.02599-10. Epub 2011 May 27. Appl Environ Microbiol. 2011. Retraction in: Appl Environ Microbiol. 2018 Jan 31;84(4):e02429-17. doi: 10.1128/AEM.02429-17. PMID: 21622797 Free PMC article. Retracted.
-
Engineering vesicle trafficking improves the extracellular activity and surface display efficiency of cellulases in Saccharomyces cerevisiae.Biotechnol Biofuels. 2017 Feb 27;10:53. doi: 10.1186/s13068-017-0738-8. eCollection 2017. Biotechnol Biofuels. 2017. PMID: 28261326 Free PMC article.
-
Engineering microbial factories for synthesis of value-added products.J Ind Microbiol Biotechnol. 2011 Aug;38(8):873-90. doi: 10.1007/s10295-011-0970-3. Epub 2011 Apr 28. J Ind Microbiol Biotechnol. 2011. PMID: 21526386 Free PMC article. Review.
-
A combined cell-consortium approach for lignocellulose degradation by specialized Lactobacillus plantarum cells.Biotechnol Biofuels. 2014 Jul 24;7:112. doi: 10.1186/1754-6834-7-112. eCollection 2014. Biotechnol Biofuels. 2014. PMID: 25788977 Free PMC article.
-
The Electrosome: A Surface-Displayed Enzymatic Cascade in a Biofuel Cell's Anode and a High-Density Surface-Displayed Biocathodic Enzyme.Nanomaterials (Basel). 2017 Jun 23;7(7):153. doi: 10.3390/nano7070153. Nanomaterials (Basel). 2017. PMID: 28644390 Free PMC article.
References
-
- Bayer, E. A., J. P. Belaich, Y. Shoham, and R. Lamed. 2004. The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides. Annu. Rev. Microbiol. 58:521-554. - PubMed
-
- Bayer, E. A., R. Lamed, and M. E. Himmel. 2007. The potential of cellulases and cellulosomes for cellulosic waste management. Curr. Opin. Biotechnol. 18:237-245. - PubMed
-
- Boder, E. T., J. R. Bill, A. W. Nields, P. C. Marrack, and J. W. Kappler. 2005. Yeast surface display of a noncovalent MHC class II heterodimer complexed with antigenic peptide. Biotechnol. Bioeng. 92:485-491. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

