The Drosophila nuclear receptors DHR3 and betaFTZ-F1 control overlapping developmental responses in late embryos
- PMID: 20023167
- PMCID: PMC2796934
- DOI: 10.1242/dev.042036
The Drosophila nuclear receptors DHR3 and betaFTZ-F1 control overlapping developmental responses in late embryos
Abstract
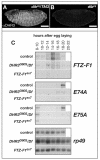
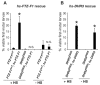
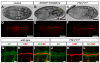
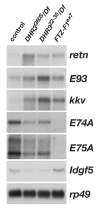
Studies of the onset of metamorphosis have identified an ecdysone-triggered transcriptional cascade that consists of the sequential expression of the transcription-factor-encoding genes DHR3, betaFTZ-F1, E74A and E75A. Although the regulatory interactions between these genes have been well characterized by genetic and molecular studies over the past 20 years, their developmental functions have remained more poorly understood. In addition, a transcriptional sequence similar to that observed in prepupae is repeated before each developmental transition in the life cycle, including mid-embryogenesis and the larval molts. Whether the regulatory interactions between DHR3, betaFTZ-F1, E74A and E75A at these earlier stages are similar to those defined at the onset of metamorphosis, however, is unknown. In this study, we turn to embryonic development to address these two issues. We show that mid-embryonic expression of DHR3 and betaFTZ-F1 is part of a 20-hydroxyecdysone (20E)-triggered transcriptional cascade similar to that seen in mid-prepupae, directing maximal expression of E74A and E75A during late embryogenesis. In addition, DHR3 and betaFTZ-F1 exert overlapping developmental functions at the end of embryogenesis. Both genes are required for tracheal air filling, whereas DHR3 is required for ventral nerve cord condensation and betaFTZ-F1 is required for proper maturation of the cuticular denticles. Rescue experiments support these observations, indicating that DHR3 has essential functions independent from those of betaFTZ-F1. DHR3 and betaFTZ-F1 also contribute to overlapping transcriptional responses during embryogenesis. Taken together, these studies define the lethal phenotypes of DHR3 and betaFTZ-F1 mutants, and provide evidence for functional bifurcation in the 20E-responsive transcriptional cascade.
Figures



References
-
- Arnone M. I., Davidson E. H. (1997). The hardwiring of development: organization and function of genomic regulatory systems. Development 124, 1851-1864 - PubMed
-
- Baehrecke E. H., Thummel C. S. (1995). The Drosophila E93 gene from the 93F early puff displays stage- and tissue-specific regulation by 20-hydroxyecdysone. Dev. Biol. 171, 85-97 - PubMed
-
- Bender M., Imam F. B., Talbot W. S., Ganetzky B., Hogness D. S. (1997). Drosophila ecdysone receptor mutations reveal functional differences among receptor isoforms. Cell 91, 777-788 - PubMed
-
- Bialecki M., Shilton A., Fichtenberg C., Segraves W. A., Thummel C. S. (2002). Loss of the ecdysteroid-inducible E75A orphan nuclear receptor uncouples molting from metamorphosis in Drosophila. Dev. Cell 3, 209-220 - PubMed
-
- Broadus J., McCabe J. R., Endrizzi B., Thummel C. S., Woodard C. T. (1999). The Drosophila beta FTZ-F1 orphan nuclear receptor provides competence for stage-specific responses to the steroid hormone ecdysone. Mol. Cell 3, 143-149 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

