Evidence for a role of vertebrate Disp1 in long-range Shh signaling
- PMID: 20023168
- PMCID: PMC2796928
- DOI: 10.1242/dev.043547
Evidence for a role of vertebrate Disp1 in long-range Shh signaling
Abstract
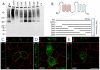
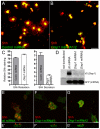
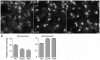
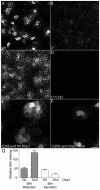
Dispatched 1 (Disp1) encodes a twelve transmembrane domain protein that is required for long-range sonic hedgehog (Shh) signaling. Inhibition of Disp1 function, both by RNAi or dominant-negative constructs, prevents secretion and results in the accumulation of Shh in source cells. Measuring the Shh response in neuralized embryoid bodies (EBs) derived from embryonic stem (ES) cells, with or without Disp1 function, demonstrates an additional role for Disp1 in cells transporting Shh. Co-cultures with Shh-expressing cells revealed a significant reduction in the range of the contact-dependent Shh response in Disp1(-/-) neuralized EBs. These observations support a dual role for Disp1, not only in the secretion of Shh from the source cells, but also in the subsequent transport of Shh through tissue.
Figures
Similar articles
-
Mouse Disp1 is required in sonic hedgehog-expressing cells for paracrine activity of the cholesterol-modified ligand.Development. 2005 Jan;132(1):133-42. doi: 10.1242/dev.01563. Epub 2004 Dec 2. Development. 2005. PMID: 15576405
-
Dose dependency of Disp1 and genetic interaction between Disp1 and other hedgehog signaling components in the mouse.Development. 2004 Aug;131(16):4021-33. doi: 10.1242/dev.01257. Epub 2004 Jul 21. Development. 2004. Retraction in: Development. 2005 Dec;132(24):5615. doi: 10.1242/dev.132.24.5615. PMID: 15269168 Retracted.
-
Disp1 regulates growth of mammalian long bones through the control of Ihh distribution.Dev Biol. 2008 May 15;317(2):480-5. doi: 10.1016/j.ydbio.2008.02.039. Epub 2008 Mar 4. Dev Biol. 2008. PMID: 18395198 Free PMC article.
-
The notch response inhibitor DAPT enhances neuronal differentiation in embryonic stem cell-derived embryoid bodies independently of sonic hedgehog signaling.Dev Dyn. 2007 Mar;236(3):886-92. doi: 10.1002/dvdy.21083. Dev Dyn. 2007. PMID: 17295317
-
Dispatched uses Na+ flux to power release of lipid-modified Hedgehog.Nature. 2021 Nov;599(7884):320-324. doi: 10.1038/s41586-021-03996-0. Epub 2021 Oct 27. Nature. 2021. PMID: 34707294 Free PMC article.
Cited by
-
Quantitative analyses link modulation of sonic hedgehog signaling to continuous variation in facial growth and shape.Development. 2010 Oct;137(20):3405-9. doi: 10.1242/dev.052340. Epub 2010 Sep 8. Development. 2010. PMID: 20826528 Free PMC article.
-
The acid-secreting parietal cell as an endocrine source of Sonic Hedgehog during gastric repair.Endocrinology. 2013 Dec;154(12):4627-39. doi: 10.1210/en.2013-1483. Epub 2013 Oct 3. Endocrinology. 2013. PMID: 24092639 Free PMC article.
-
Dispatching Sonic Hedgehog: Molecular Mechanisms Controlling Deployment.Trends Cell Biol. 2019 May;29(5):385-395. doi: 10.1016/j.tcb.2019.02.005. Epub 2019 Mar 6. Trends Cell Biol. 2019. PMID: 30852081 Free PMC article. Review.
-
Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function.Dev Cell. 2011 Jun 14;20(6):775-87. doi: 10.1016/j.devcel.2011.04.018. Dev Cell. 2011. PMID: 21664576 Free PMC article.
-
Computational Model of Secondary Palate Fusion and Disruption.Chem Res Toxicol. 2017 Apr 17;30(4):965-979. doi: 10.1021/acs.chemrestox.6b00350. Epub 2017 Jan 20. Chem Res Toxicol. 2017. PMID: 28045533 Free PMC article.
References
-
- Albright T. D., Jessell T. M., Kandel E. R., Posner M. I. (2000). Neural science: a century of progress and the mysteries that remain. Neuron 25Suppl., S1-S55 - PubMed
-
- Burke R., Nellen D., Bellotto M., Hafen E., Senti K.-A., Dickson B. J., Basler K. (1999). Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 99, 803-815 - PubMed
-
- Callejo A., Torroja C., Quijada L., Guerrero I. (2006). Hedgehog lipid modifications are required for Hedgehog stabilization in the extracellular matrix. Development 133, 471-483 - PubMed
-
- Caspary T., Garcia-Garcia M. J., Huangfu D., Eggenschwiler J. T., Wyler M. R., Rakeman A. S., Alcorn H. L., Anderson K. V. (2002). Mouse Dispatched homolog1 is required for long-range, but not juxtacrine, Hh signaling. Curr. Biol. 12, 1628-1632 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

