Transforming growth factor-beta modulates the expression of nitric oxide signaling enzymes in the injured developing lung and in vascular smooth muscle cells
- PMID: 20023176
- PMCID: PMC2838670
- DOI: 10.1152/ajplung.00181.2009
Transforming growth factor-beta modulates the expression of nitric oxide signaling enzymes in the injured developing lung and in vascular smooth muscle cells
Abstract
Nitric oxide signaling has an important role in regulating pulmonary development and function. Expression of soluble guanylate cyclase (sGC) and cGMP-dependent protein kinase I (PKGI), both critical mediators of nitric oxide (NO) signaling, is diminished in the injured newborn lung through unknown mechanisms. Recent studies suggest that excessive transforming growth factor-beta (TGF-beta) activity inhibits injured newborn lung development. To explore mechanisms that regulate pulmonary NO signaling, we tested whether TGF-beta decreases sGC and PKGI expression in the injured developing lung and pulmonary vascular smooth muscle cells (SMC). We found that chronic oxygen-induced lung injury decreased pulmonary sGCalpha(1) and PKGI immunoreactivity in mouse pups and that exposure to a TGF-beta-neutralizing antibody prevented this reduction of sGC and PKGI protein expression. In addition, TGF-beta(1) decreased expression of NO signaling enzymes in freshly isolated pulmonary microvascular SMC/myofibroblasts, suggesting that TGF-beta has a direct role in modulating NO signaling in the pup lung. Moreover, TGF-beta(1) decreased sGC and PKGI expression in pulmonary artery and aortic SMC from adult rats and mice, suggesting a general role for TGF-beta in modulating NO signaling in vascular SMC. Although other cytokines decrease sGC mRNA stability, TGF-beta did not modulate sGCalpha(1) or PKGIbeta mRNA turnover in vascular SMC. These studies indicate for the first time that TGF-beta decreases NO signaling enzyme expression in the injured developing lung and pulmonary vascular SMC. Moreover, they suggest that TGF-beta-neutralizing molecules might counteract the effects of injury on NO signaling in the newborn lung.
Figures

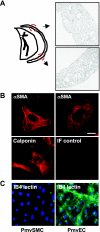
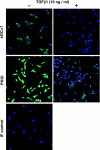
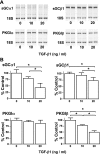


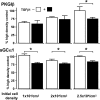
Similar articles
-
Soluble guanylate cyclase modulates alveolarization in the newborn lung.Am J Physiol Lung Cell Mol Physiol. 2013 Oct 15;305(8):L569-81. doi: 10.1152/ajplung.00401.2012. Epub 2013 Aug 9. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23934926 Free PMC article.
-
Regulation of the expression of soluble guanylyl cyclase by reactive oxygen species.Br J Pharmacol. 2007 Apr;150(8):1084-91. doi: 10.1038/sj.bjp.0707179. Epub 2007 Mar 5. Br J Pharmacol. 2007. PMID: 17339839 Free PMC article.
-
Transforming growth factor-β downregulates sGC subunit expression in pulmonary artery smooth muscle cells via MEK and ERK signaling.Am J Physiol Lung Cell Mol Physiol. 2019 Jan 1;316(1):L20-L34. doi: 10.1152/ajplung.00319.2018. Epub 2018 Sep 27. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 30260287 Free PMC article.
-
Molecular variants of soluble guanylyl cyclase affecting cardiovascular risk.Circ J. 2015;79(3):463-9. doi: 10.1253/circj.CJ-15-0025. Epub 2015 Feb 6. Circ J. 2015. PMID: 25746521 Review.
-
Structure and regulation of soluble guanylate cyclase.Annu Rev Biochem. 2012;81:533-59. doi: 10.1146/annurev-biochem-050410-100030. Epub 2012 Feb 9. Annu Rev Biochem. 2012. PMID: 22404633 Review.
Cited by
-
Soluble guanylate cyclase modulators blunt hyperoxia effects on calcium responses of developing human airway smooth muscle.Am J Physiol Lung Cell Mol Physiol. 2015 Sep 15;309(6):L537-42. doi: 10.1152/ajplung.00232.2015. Epub 2015 Aug 7. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26254425 Free PMC article.
-
Soluble guanylate cyclase modulates alveolarization in the newborn lung.Am J Physiol Lung Cell Mol Physiol. 2013 Oct 15;305(8):L569-81. doi: 10.1152/ajplung.00401.2012. Epub 2013 Aug 9. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23934926 Free PMC article.
-
Transforming growth factor-β stimulates Smad1/5 signaling in pulmonary artery smooth muscle cells and fibroblasts of the newborn mouse through ALK1.Am J Physiol Lung Cell Mol Physiol. 2017 Sep 1;313(3):L615-L627. doi: 10.1152/ajplung.00079.2017. Epub 2017 Jun 22. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28642261 Free PMC article.
-
Polygenic Causes of Congenital Diaphragmatic Hernia Produce Common Lung Pathologies.Am J Pathol. 2016 Oct;186(10):2532-43. doi: 10.1016/j.ajpath.2016.07.006. Epub 2016 Aug 24. Am J Pathol. 2016. PMID: 27565037 Free PMC article. Review.
-
Challenging the Norm: The Unrecognized Impact of Soluble Guanylyl Cyclase Subunits in Cancer.Int J Mol Sci. 2024 Sep 19;25(18):10053. doi: 10.3390/ijms251810053. Int J Mol Sci. 2024. PMID: 39337539 Free PMC article. Review.
References
-
- Afshar S, Gibson LL, Yuhanna IS, Sherman TS, Kerecman JD, Grubb PH, Yoder BA, McCurnin DC, Shaul PW. Pulmonary NO synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am J Physiol Lung Cell Mol Physiol 284: L749–L758, 2003 - PubMed
-
- Alejandre-Alcázar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B, Köbrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-β/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007 - PubMed
-
- Ambalavanan N, Nicola T, Hagood J, Bulger A, Serra R, Murphy-Ullrich J, Oparil S, Chen YF. Transforming growth factor-β signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 295: L86–L95, 2008 - PMC - PubMed
-
- Behrends S, Kempfert J, Mietens A, Koglin M, Scholz H, Middendorff R. Developmental changes of nitric oxide-sensitive guanylyl cyclase expression in pulmonary arteries. Biochem Biophys Res Commun 283: 883–887, 2001 - PubMed
-
- Black SM, Johengen MJ, Soifer SJ. Coordinated regulation of genes of the nitric oxide and endothelin pathways during the development of pulmonary hypertension in fetal lambs. Pediatr Res 44: 821–830, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

