Bile alcohols function as the ligands of membrane-type bile acid-activated G protein-coupled receptor
- PMID: 20023205
- PMCID: PMC3035506
- DOI: 10.1194/jlr.M004051
Bile alcohols function as the ligands of membrane-type bile acid-activated G protein-coupled receptor
Abstract
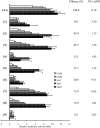
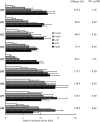
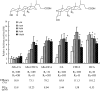
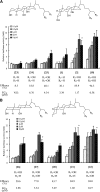
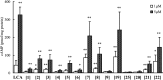
TGR5 is a G protein-coupled receptor that is activated by bile acids, resulting in an increase in cAMP levels and the subsequent modulation of energy expenditure in brown adipose tissue and muscle. Therefore, the development of a TGR5-specific agonist could lead to the prevention and treatment of various metabolic disorders related to obesity. In the present study, we evaluated the ability of bile alcohols, which are structurally and physiologically similar to bile acids and are produced as the end products of cholesterol catabolism in evolutionarily primitive vertebrates, to act as TGR5 agonists. In a cell-based reporter assay and a cAMP production assay performed in vitro, most bile alcohols with a side chain containing hydroxyl group(s) were highly efficacious agonists for TGR5 comparable to its most potent ligand in the naturally occurring bile acid, lithocholic acid. However, the abilities of the bile alcohols to activate TGR5 varied with the position and number of the hydroxyl substituent in the side chain. Additionally, the conformation of the steroidal nucleus of bile alcohols is also important for its activity as a TGR5 agonist. Thus, we have provided new insights into the structure-activity relationships of bile alcohols as TGR5 agonists.
Figures

References
-
- Wang H., Chen J., Hollister K., Sowers L. C., Forman B. M. 1999. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 3: 543–553. - PubMed
-
- Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., et al. 1999. Bile acids: natural ligands for an orphan nuclear receptor. Science. 284: 1365–1368. - PubMed
-
- Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., Hull M. V., Lustig K. D., Mangelsdorf D. J., Shan B. 1999. Identification of a nuclear receptor for bile acids. Science. 284: 1362–1365. - PubMed
-
- Everson G. T. 1987. Steady-state kinetics of serum bile acids in healthy human subjects: single and dual isotope techniques using stable isotopes and mass spectrometry. J. Lipid Res. 28: 238–252. - PubMed
-
- Ho K. J. 1976. Circadian distribution of bile acid in the enterohepatic circulatory system in hamsters. J. Lipid Res. 17: 600–604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

