Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma
- PMID: 20023215
- PMCID: PMC2858478
- DOI: 10.1182/blood-2009-08-231613
Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma
Abstract
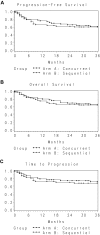
Rituximab plus intravenous bolus chemotherapy is a standard treatment for immunocompetent patients with B-cell non-Hodgkin lymphoma (NHL). Some studies have suggested that rituximab is associated with excessive toxicity in HIV-associated NHL, and that infusional chemotherapy may be more effective. We performed a randomized phase 2 trial of rituximab (375 mg/m(2)) given either concurrently before each infusional etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone (EPOCH) chemotherapy cycle or sequentially (weekly for 6 weeks) after completion of all chemotherapy in HIV-associated NHL. EPOCH consisted of a 96-hour intravenous infusion of etoposide, doxorubicin, and vincristine plus oral prednisone followed by intravenous bolus cyclophosphamide given every 21 days for 4 to 6 cycles. In the concurrent arm, 35 of 48 evaluable patients (73%; 95% confidence interval, 58%-85%) had a complete response. In the sequential arm, 29 of 53 evaluable patients (55%; 95% confidence interval, 41%-68%) had a complete response. The primary efficacy endpoint was met for the concurrent arm only. Toxicity was comparable in the 2 arms, although patients with a baseline CD4 count less than 50/microL had a high infectious death rate in the concurrent arm. We conclude that concurrent rituximab plus infusional EPOCH is an effective regimen for HIV-associated lymphoma.
Trial registration: ClinicalTrials.gov NCT00049036.
Figures
Comment in
-
HIV-associated lymphoma.Blood. 2010 Apr 15;115(15):2986-7. doi: 10.1182/blood-2010-01-262717. Blood. 2010. PMID: 20395421 No abstract available.
Similar articles
-
Venetoclax with dose-adjusted EPOCH-R as initial therapy for patients with aggressive B-cell lymphoma: a single-arm, multicentre, phase 1 study.Lancet Haematol. 2021 Nov;8(11):e818-e827. doi: 10.1016/S2352-3026(21)00273-8. Epub 2021 Oct 8. Lancet Haematol. 2021. PMID: 34634256 Clinical Trial.
-
Pooled analysis of AIDS malignancy consortium trials evaluating rituximab plus CHOP or infusional EPOCH chemotherapy in HIV-associated non-Hodgkin lymphoma.Cancer. 2012 Aug 15;118(16):3977-83. doi: 10.1002/cncr.26723. Epub 2011 Dec 16. Cancer. 2012. PMID: 22180164 Free PMC article.
-
Randomized phase II study of concurrent and sequential combinations of rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) chemotherapy in untreated indolent B-cell non-Hodgkin lymphoma: 7-year follow-up results.Cancer Sci. 2010 Dec;101(12):2579-85. doi: 10.1111/j.1349-7006.2010.01703.x. Cancer Sci. 2010. PMID: 20942866 Free PMC article. Clinical Trial.
-
Treatment factors affecting outcomes in HIV-associated non-Hodgkin lymphomas: a pooled analysis of 1546 patients.Blood. 2013 Nov 7;122(19):3251-62. doi: 10.1182/blood-2013-04-498964. Epub 2013 Sep 6. Blood. 2013. PMID: 24014242 Free PMC article. Review.
-
Rituximab for HIV-associated lymphoma: weighing the benefits and risks.Curr Opin Oncol. 2005 Sep;17(5):462-5. doi: 10.1097/01.cco.0000172824.78318.b9. Curr Opin Oncol. 2005. PMID: 16093796 Review.
Cited by
-
HIV-associated hematologic malignancies: Experience from a Tertiary Cancer Center in India.Indian J Med Paediatr Oncol. 2016 Jul-Sep;37(3):141-5. doi: 10.4103/0971-5851.190355. Indian J Med Paediatr Oncol. 2016. PMID: 27688606 Free PMC article.
-
Safety and efficacy of an oncolytic viral strategy using bortezomib with ICE/R in relapsed/refractory HIV-positive lymphomas.Blood Adv. 2018 Dec 26;2(24):3618-3626. doi: 10.1182/bloodadvances.2018022095. Blood Adv. 2018. PMID: 30573564 Free PMC article. Clinical Trial.
-
Multicentric Castleman's disease and HIV.Proc (Bayl Univ Med Cent). 2014 Jan;27(1):28-30. doi: 10.1080/08998280.2014.11929044. Proc (Bayl Univ Med Cent). 2014. PMID: 24381398 Free PMC article.
-
Predictive Value of Cytokines and Immune Activation Biomarkers in AIDS-Related Non-Hodgkin Lymphoma Treated with Rituximab plus Infusional EPOCH (AMC-034 trial).Clin Cancer Res. 2016 Jan 15;22(2):328-36. doi: 10.1158/1078-0432.CCR-14-0466. Epub 2015 Sep 17. Clin Cancer Res. 2016. PMID: 26384320 Free PMC article. Clinical Trial.
-
Efficacy and safety of different chemotherapy regimens combined with thalidomide in the treatment of diagnosed HIV-associated diffuse large B-cell lymphoma.Leuk Res Rep. 2024 Mar 9;21:100450. doi: 10.1016/j.lrr.2024.100450. eCollection 2024. Leuk Res Rep. 2024. PMID: 38516379 Free PMC article. Review.
References
-
- Maloney DG, Grillo-Lopez AJ, White CA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997;90(6):2188–2195. - PubMed
-
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. - PubMed
-
- Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121–3127. - PubMed
-
- Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379–391. - PubMed
-
- Thomas DA, Faderl S, O'Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106(7):1569–1580. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous