Platelet-derived growth factor BB mediates the tropism of human mesenchymal stem cells for malignant gliomas
- PMID: 20023545
- PMCID: PMC2811094
- DOI: 10.1227/01.NEU.0000363149.58885.2E
Platelet-derived growth factor BB mediates the tropism of human mesenchymal stem cells for malignant gliomas
Abstract
Objective: Bone marrow-derived human mesenchymal stem cells (hMSCs) are capable of localizing to gliomas after systemic delivery and can be used in glioma therapy. However, the mechanism underlying the tropism of hMSCs for gliomas remains unclear. In vitro studies suggest that platelet-derived growth factor BB (PDGF-BB) may mediate this tropism. However, a causal role of PDGF-BB has not been demonstrated in vivo. Therefore, we tested the hypothesis that PDGF-BB mediates the attraction of hMSCs to gliomas in vitro and in vivo.
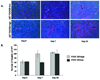
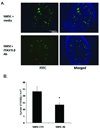
Methods: U87 or LN229 glioma cells were transfected with plasmids encoding human PDGF-B. Stable transfected clones that secreted large amounts of PDFG-BB and clones that produced low levels of PDGF were chosen. In vitro migration of hMSCs toward PDGF-B or conditioned media from high- and low-secreting PDGF-B tumor cells was assessed using Matrigel invasion assays. For in vivo localization studies, hMSCs were tracked by bioluminescence imaging (BLI) after transduction with an adenovirus containing luciferase cDNA. In other studies, hMSCs were labeled with green fluorescent protein (gfp) and analyzed for intratumoral localization by immunohistochemistry.
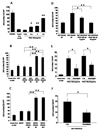
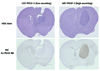
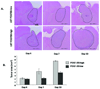
Results: In vitro invasion assays showed that significantly more hMSCs migrated toward glioma cells engineered to secrete high levels of PDGF-BB compared with low-secreting gliomas. Anti-PDGF-BB-neutralizing antibody abrogated this increase in migration. Pretreatment of hMSCs with inhibitory antibodies against PDGF receptor-beta also reduced hMSC migration. To demonstrate that PDGF-BB mediates the localization of hMSCs in vivo, hMSCs-Ad-Luc were injected into the carotid artery of mice harboring orthotopic 7-day-old U87-PDGF-BB-high secreting or U87-PDGF-BB-low secreting xenografts and analyzed by BLI. Statistically significant increases in hMSCs were seen within PDGF-BB-high xenografts compared with PDGF-BB-low xenografts. To control for PDGF-BB-induced differences in tumor size and vascularity, gfp-labeled hMSCs were injected into the carotid arteries of animals harboring 4-day old PDGF-BB-high secreting xenografts or 7-day old PDGF-BB-low secreting xenografts. At these times tumors had similar size and vessel density. Statistically significant more hMSCs localized to PDGF-BB-high secreting xenografts compared with PDGF-BB-low secreting xenografts. Pretreatment of hMSCs with anti-PDGFR-beta-inhibitory antibodies decreased the localization of hMSCs in this intracranial model.
Conclusion: PDGF-BB increases the attraction of hMSCs for gliomas in vitro and in vivo, and this tropism is mediated via PDGF-beta receptors on hMSCs. These findings can be exploited for advancing hMSC treatment.
Figures




References
-
- Birnbaum T, Roider J, Schankin CJ, Padovan CS, Schichor C, Goldbrunner R, Straube A. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol. 2007;83:241–247. - PubMed
-
- Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259–264. - PubMed
-
- Cheng P, Gao ZQ, Liu YH, Xue YX. Platelet-derived growth factor BB promotes the migration of bone marrow-derived mesenchymal stem cells towards C6 glioma and up-regulates the expression of intracellular adhesion molecule-1. Neuroscience letters. 2009;451:52–56. - PubMed
-
- Fiedler J, Etzel N, Brenner RE. To go or not to go: Migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. Journal of cellular biochemistry. 2004;93:990–998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

