Deficiency of Bid protein reduces sepsis-induced apoptosis and inflammation, while improving septic survival
- PMID: 20023601
- PMCID: PMC2909339
- DOI: 10.1097/SHK.0b013e3181cf70fb
Deficiency of Bid protein reduces sepsis-induced apoptosis and inflammation, while improving septic survival
Abstract
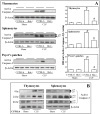
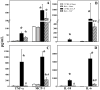
Increased apoptotic cell death is believed to play a pathological role in patients with sepsis and experimental animals. Apoptosis can be induced by either a cell death receptor (extrinsic) or a mitochondrial (intrinsic) pathway. Bid, a proapoptotic member of the Bcl-2 family, is thought to mediate the cross talk between the extrinsic and intrinsic pathways of apoptosis; however, little is known about the action of Bid in the development of apoptosis and organ-specific tissue damage/cell death as seen in polymicrobial sepsis. Our results show that after the onset of sepsis, tBid (the active form of Bid) is significantly increased in mitochondrial fractions of the thymus, spleen, Peyer patches, and liver, and that Fas or FasL deficiency blocks Bid activation in various tissues after septic challenge. Increased Bid activation is correlated with increased active caspase-3, caspase-9, and apoptosis during sepsis. Bid-deficient mice exhibit significantly reduced apoptosis in the thymus, spleen, and Peyer patches compared with background mice after sepsis. Furthermore, Bid-deficient mice had significantly reduced systemic and local inflammatory cytokine levels and improved survival after sepsis. These data support not only the contribution of Bid to sepsis-induced apoptosis and the onset of septic morbidity/mortality, but also the existence of a bridge between extrinsic apoptotic signals, e.g., FasL:Fas, TNF:TNFR, and so on, and the intrinsic mitochondrial pathway via Bid-tBid activation during sepsis.
Figures









Similar articles
-
The caspase-8/Bid/cytochrome c axis links signals from death receptors to mitochondrial reactive oxygen species production.Free Radic Biol Med. 2017 Nov;112:567-577. doi: 10.1016/j.freeradbiomed.2017.09.001. Epub 2017 Sep 6. Free Radic Biol Med. 2017. PMID: 28888620
-
CD8+ T cells promote inflammation and apoptosis in the liver after sepsis: role of Fas-FasL.Am J Pathol. 2007 Jul;171(1):87-96. doi: 10.2353/ajpath.2007.061099. Am J Pathol. 2007. PMID: 17591956 Free PMC article.
-
Bcl-2 family member Bfl-1/A1 sequesters truncated bid to inhibit is collaboration with pro-apoptotic Bak or Bax.J Biol Chem. 2002 Jun 21;277(25):22781-8. doi: 10.1074/jbc.M201469200. Epub 2002 Apr 19. J Biol Chem. 2002. PMID: 11929871
-
Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways.Cell Res. 2000 Sep;10(3):161-7. doi: 10.1038/sj.cr.7290045. Cell Res. 2000. PMID: 11032168 Review.
-
Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra.J Neuropathol Exp Neurol. 2003 Apr;62(4):329-39. doi: 10.1093/jnen/62.4.329. J Neuropathol Exp Neurol. 2003. PMID: 12722825 Review.
Cited by
-
Revisiting caspases in sepsis.Cell Death Dis. 2014 Nov 20;5(11):e1526. doi: 10.1038/cddis.2014.488. Cell Death Dis. 2014. PMID: 25412304 Free PMC article. Review.
-
Immunosuppression after sepsis: systemic inflammation and sepsis induce a loss of naïve T-cells but no enduring cell-autonomous defects in T-cell function.PLoS One. 2014 Dec 26;9(12):e115094. doi: 10.1371/journal.pone.0115094. eCollection 2014. PLoS One. 2014. PMID: 25541945 Free PMC article.
-
Metabolic Inflammatory Complex in Sepsis: Septic Cachexia as a Novel Potential Therapeutic Target.Shock. 2017 Dec;48(6):600-609. doi: 10.1097/SHK.0000000000000906. Shock. 2017. PMID: 28520694 Free PMC article. Review.
-
Platelets induce apoptosis during sepsis in a contact-dependent manner that is inhibited by GPIIb/IIIa blockade.PLoS One. 2012;7(7):e41549. doi: 10.1371/journal.pone.0041549. Epub 2012 Jul 26. PLoS One. 2012. PMID: 22844498 Free PMC article.
-
In vivo evaluation of the protective effects of arjunolic acid against lipopolysaccharide-induced septic myocardial injury.PeerJ. 2022 Feb 16;10:e12986. doi: 10.7717/peerj.12986. eCollection 2022. PeerJ. 2022. PMID: 35190789 Free PMC article.
References
-
- Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukocyte Biol. 1994;56:672–686. - PubMed
-
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. - PubMed
-
- Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmac Ther. 2001;92:57–70. - PubMed
-
- Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. - PubMed
-
- Ayala A, Chung CS, Chaudry IH. Lymphocyte apoptosis in sepsis. Sepsis. 1998;2:55–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

