Strong cooperativity between subunits in voltage-gated proton channels
- PMID: 20023639
- PMCID: PMC2935852
- DOI: 10.1038/nsmb.1739
Strong cooperativity between subunits in voltage-gated proton channels
Abstract
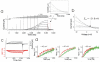
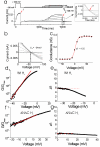
Voltage-activated proton (Hv) channels are essential components in the innate immune response. Hv channels are dimeric proteins with one proton permeation pathway per subunit. It is unknown how Hv channels are activated by voltage and whether there is any cooperation between subunits during voltage activation. Using cysteine accessibility measurements and voltage-clamp fluorometry, we show data consistent with the possibility that the fourth transmembrane segment S4 functions as the voltage sensor in Ciona intestinalis Hv channels. Unexpectedly, in a dimeric Hv channel, the S4 in both subunits must move to activate the two proton permeation pathways. In contrast, if Hv subunits are prevented from dimerizing, the movement of a single S4 is sufficient to activate the proton permeation pathway in a subunit. These results indicate strong cooperativity between subunits in dimeric Hv channels.
Figures



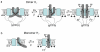
References
-
- Decoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. - PubMed
-
- Okamura Y. Biodiversity of voltage sensor domain proteins. Pflugers Arch. 2007;454:361–371. - PubMed
-
- Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. - PubMed
-
- Hille B. Ion Channels of Excitable Membranes. Sinauer Associates, INC; Sunderland, MA: 2001.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

