Systemic in vivo distribution of activatable cell penetrating peptides is superior to that of cell penetrating peptides
- PMID: 20023744
- PMCID: PMC2796831
- DOI: 10.1039/b904878b
Systemic in vivo distribution of activatable cell penetrating peptides is superior to that of cell penetrating peptides
Abstract
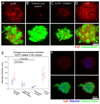
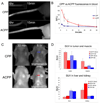
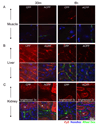
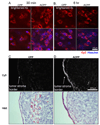
Cell penetrating peptides (CPPs) have been developed as vehicles for payload delivery into cells in culture and in animals. However several biologic features limit their usefulness in living animals. Activatable cell penetrating peptides (ACPPs) are polycationic CPPs whose adsorption and cellular uptake are minimized by a covalently attached polyanionic inhibitory domain. Cleavage of the linker connecting the polyanionic and polycationic domains by specific proteases (tumor associated matrix metalloproteases discussed herein) dissociates the polyanion and enables the cleaved ACPP to enter cells. In contrast to their CPP counterpart, ACPPs are relatively nonadherent and distributed uniformly to normal tissues. While nonaarginine (r(9)) CPP administered intravenously into mice initially bind to the local vasculature and redistribute to the liver, where >90% of the injected dose accumulates 30 min after injection. Regardless of the presence of the polyanionic inhibitory domain, confocal imaging of live tissues reveals that the majority of the ACPP and CPP remain in punctate organelles, presumably endosomes. Therefore further improvements in the efficiency of delivery to the cytosol and nucleus are necessary. In addition to improved target specificity, a major advantage of ACPPs over CPPs for potential clinical applications is reduced toxicity. Systemically administered r(9) CPP causes acute toxicity in mice at a dose 4-fold lower than the MMP cleavable ACPP, a complication not observed with an uncleavable ACPP presumably because the polycationic charge remains masked systemically. These data suggest that ACPPs have greater potential than CPPs for systemic delivery of imaging and therapeutic agents.
Figures

References
-
- Torchilin VP. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Adv. Drug Deliv. Rev. 2008;60:548–558. - PubMed
-
- Patel LN, Zaro JL, Shen WC. Cell penetrating peptides: intracellular pathways and pharmaceutical perspectives. Pharm. Res. 2007;24:1977–1992. - PubMed
-
- Lundberg M, Johansson M. Is VP22 nuclear homing an artifact? Nat. Biotechnol. 2001;19:713–714. - PubMed
-
- Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 2003;278:585–590. - PubMed
-
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

