Non-traditional platinum compounds for improved accumulation, oral bioavailability, and tumor targeting
- PMID: 20023892
- PMCID: PMC2800312
- DOI: 10.1039/b913896j
Non-traditional platinum compounds for improved accumulation, oral bioavailability, and tumor targeting
Abstract
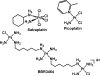
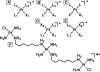
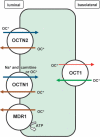
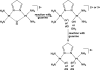
The five platinum anticancer compounds currently in clinical use conform to structure-activity relationships formulated (M. J. Cleare and J. D. Hoeschele, Bioinorg. Chem., 1973, 2, 187-210) shortly after the discovery that cis-diamminedichloroplatinum(II), cisplatin, has antitumor activity in mice. These compounds are neutral platinum(II) species with two am(m)ine ligands or one bidentate chelating diamine and two additional ligands that can be replaced by water through aquation reactions. The resulting cations ultimately form bifunctional adducts on DNA. Information about the chemistry of these platinum compounds and correlations of their structures with anticancer activity have provided guidance for the design of novel anticancer drug candidates based on the proposed mechanisms of action. This article discusses advances in the synthesis and evaluation of such non-traditional platinum compounds, including cationic and tumor-targeting constructs.
Figures



Similar articles
-
DNA binding by antitumor trans-[PtCl2(NH3)(thiazole)]. Protein recognition and nucleotide excision repair of monofunctional adducts.Biochemistry. 2003 Jan 28;42(3):792-800. doi: 10.1021/bi026614t. Biochemistry. 2003. PMID: 12534292
-
Structure, solution chemistry, antiproliferative actions and protein binding properties of non-conventional platinum(II) compounds with sulfur and phosphorus donors.Dalton Trans. 2011 Mar 7;40(9):2006-16. doi: 10.1039/c0dt00845a. Epub 2011 Jan 6. Dalton Trans. 2011. PMID: 21212880
-
Cellular accumulation and DNA platination of two new platinum(II) anticancer compounds based on anthracene derivatives as carrier ligands.J Inorg Biochem. 2009 May;103(5):791-6. doi: 10.1016/j.jinorgbio.2009.02.005. Epub 2009 Feb 27. J Inorg Biochem. 2009. PMID: 19303143
-
DNA modifications by antitumor platinum and ruthenium compounds: their recognition and repair.Prog Nucleic Acid Res Mol Biol. 2002;71:1-68. doi: 10.1016/s0079-6603(02)71040-4. Prog Nucleic Acid Res Mol Biol. 2002. PMID: 12102553 Review.
-
Cisplatin and beyond: molecular mechanisms of action and drug resistance development in cancer chemotherapy.Radiol Oncol. 2019 Mar 28;53(2):148-158. doi: 10.2478/raon-2019-0018. Radiol Oncol. 2019. PMID: 30956230 Free PMC article. Review.
Cited by
-
Novel oxidatively activated agents modify DNA and are enhanced by ercc1 silencing.Chem Res Toxicol. 2012 Nov 19;25(11):2542-52. doi: 10.1021/tx300337j. Epub 2012 Oct 22. Chem Res Toxicol. 2012. PMID: 23051149 Free PMC article.
-
The Application of ATR-FTIR Spectroscopy and the Reversible DNA Conformation as a Sensor to Test the Effectiveness of Platinum(II) Anticancer Drugs.Sensors (Basel). 2018 Dec 6;18(12):4297. doi: 10.3390/s18124297. Sensors (Basel). 2018. PMID: 30563229 Free PMC article.
-
Oxidative Reactivity and Cytotoxic Properties of a Platinum(II) Complex Prepared by Outer-Sphere Amide Bond Coupling.Polyhedron. 2013 Jul 13;58:71-78. doi: 10.1016/j.poly.2012.07.097. Polyhedron. 2013. PMID: 24489429 Free PMC article.
-
Investigation of the biological and anti-cancer properties of ellagic acid-encapsulated nano-sized metalla-cages.Int J Nanomedicine. 2015 Sep 2;10 Spec Iss(Spec Iss):227-40. doi: 10.2147/IJN.S88289. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26366074 Free PMC article.
-
Oxidation of the Platinum(II) Anticancer Agent [Pt{(p-BrC6F4)NCH2CH2NEt2}Cl(py)] to Platinum(IV) Complexes by Hydrogen Peroxide.Molecules. 2023 Sep 1;28(17):6402. doi: 10.3390/molecules28176402. Molecules. 2023. PMID: 37687231 Free PMC article.
References
-
- Cleare MJ, Hoeschele JD. Bioinorg. Chem. 1973;2:187–210.
-
- Rosenberg B, VanCamp L, Trosko JE, Mansour VH. Nature. 1969;222:385–386. - PubMed
-
- Kidani YI, K. J. Med. Chem. 1978;21:1315–1318. - PubMed
-
- Rosenberg B. Met. Ions Biol. Syst. 1980;11:127–196.
-
- Campone M, Rademaker-Lakhai JM, Bennouna J, Howell SB, Nowotnik DP, Beijnen JH, Schellens JHM. Cancer Chemother. Pharmacol. 2007;60:523–533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

