Human solCD39 inhibits injury-induced development of neointimal hyperplasia
- PMID: 20024507
- PMCID: PMC2847853
- DOI: 10.1160/TH09-05-0305
Human solCD39 inhibits injury-induced development of neointimal hyperplasia
Abstract
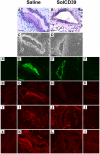
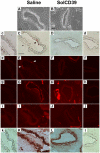
Blood platelets provide the initial response to vascular endothelial injury, becoming activated as they adhere to the injured site. Activated platelets recruit leukocytes, and initiate proliferation and migration of vascular smooth muscle cells (SMC) within the injured vessel wall, leading to development of neointimal hyperplasia. Endothelial CD39/NTPDase1 and recombinant solCD39 rapidly metabolise nucleotides, including stimulatory ADP released from activated platelets, thereby suppressing additional platelet reactivity. Using a murine model of vascular endothelial injury, we investigated whether circulating human solCD39 could reduce platelet activation and accumulation, thus abating leukocyte infiltration and neointimal formation following vascular damage. Intraperitoneally-administered solCD39 ADPase activity in plasma peaked 1 hour (h) post-injection, with an elimination half-life of 43 h. Accordingly, mice were administered solCD39 or saline 1 h prior to vessel injury, then either sacrificed 24 h post-injury or treated with solCD39 or saline (three times weekly) for an additional 18 days. Twenty-four hours post-injury, solCD39-treated mice displayed a reduction in platelet activation and recruitment, P-selectin expression, and leukocyte accumulation in the arterial lumen. Furthermore, repeated administration of solCD39 modulated the late stage of vascular injury by suppressing leukocyte deposition, macrophage infiltration and smooth muscle cell (SMC) proliferation/migration, resulting in abrogation of neointimal thickening. In contrast, injured femoral arteries of saline-injected mice exhibited massive platelet thrombus formation, marked P-selectin expression, and leukocyte infiltration. Pronounced neointimal growth with macrophage and SMC accretion was also observed (intimal-to-medial area ratio 1.56 +/- 0.34 at 19 days). Thus, systemic administration of solCD39 profoundly affects injury-induced cellular responses, minimising platelet deposition and leukocyte recruitment, and suppressing neointimal hyperplasia.
Figures



Similar articles
-
Inhibition of platelet recruitment by endothelial cell CD39/ecto-ADPase: significance for occlusive vascular diseases.Ital Heart J. 2001 Nov;2(11):824-30. Ital Heart J. 2001. PMID: 11770867
-
Effects of SolCD39, a novel inhibitor of Platelet Aggregation, on Platelet Deposition and Aggregation after PTCA in a Porcine Model.J Thromb Thrombolysis. 2005 Apr;19(2):115-22. doi: 10.1007/s11239-005-1381-y. J Thromb Thrombolysis. 2005. PMID: 16052302
-
Recombinant soluble apyrase APT102 inhibits thrombosis and intimal hyperplasia in vein grafts without adversely affecting hemostasis or re-endothelialization.J Thromb Haemost. 2017 Apr;15(4):814-825. doi: 10.1111/jth.13621. Epub 2017 Feb 23. J Thromb Haemost. 2017. PMID: 28079982 Free PMC article.
-
Metabolic control of excessive extracellular nucleotide accumulation by CD39/ecto-nucleotidase-1: implications for ischemic vascular diseases.J Pharmacol Exp Ther. 2003 Apr;305(1):9-16. doi: 10.1124/jpet.102.043729. J Pharmacol Exp Ther. 2003. PMID: 12649347 Review.
-
Role of CD39 (NTPDase-1) in thromboregulation, cerebroprotection, and cardioprotection.Semin Thromb Hemost. 2005 Apr;31(2):234-46. doi: 10.1055/s-2005-869528. Semin Thromb Hemost. 2005. PMID: 15852226 Review.
Cited by
-
In vitro Study of a Novel Stent Coating Using Modified CD39 Messenger RNA to Potentially Reduce Stent Angioplasty-Associated Complications.PLoS One. 2015 Sep 18;10(9):e0138375. doi: 10.1371/journal.pone.0138375. eCollection 2015. PLoS One. 2015. PMID: 26381750 Free PMC article.
-
Inhibition of Vascular Smooth Muscle Cell Proliferation by ENPP1: The Role of CD73 and the Adenosine Signaling Axis.Cells. 2024 Jun 29;13(13):1128. doi: 10.3390/cells13131128. Cells. 2024. PMID: 38994980 Free PMC article.
-
Nucleotide ecto-enzyme metabolic pattern and spatial distribution in calcific aortic valve disease; its relation to pathological changes and clinical presentation.Clin Res Cardiol. 2020 Feb;109(2):137-160. doi: 10.1007/s00392-019-01495-x. Epub 2019 May 29. Clin Res Cardiol. 2020. PMID: 31144065 Free PMC article.
-
Proteomic identification of biomarkers of vascular injury.Am J Transl Res. 2011 Feb;3(2):139-48. Epub 2010 Nov 21. Am J Transl Res. 2011. PMID: 21416056 Free PMC article.
-
Optimizing human apyrase to treat arterial thrombosis and limit reperfusion injury without increasing bleeding risk.Sci Transl Med. 2014 Aug 6;6(248):248ra105. doi: 10.1126/scitranslmed.3009246. Sci Transl Med. 2014. PMID: 25100739 Free PMC article.
References
-
- Roque M, Fallon JT, Badimon JJ, et al. Mouse model of femoral artery denudation injury associated with the rapid accumulation of adhesion molecules on the luminal surface and recruitment of neutrophils. Arterioscler Thromb Vasc Biol. 2000;20:335–42. - PubMed
-
- Mitra AK, Gangahar DM, Agrawal DK. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol. 2006;84:115–24. - PubMed
-
- May AE, Langer H, Seizer P, et al. Platelet-leukocyte interactions in inflammation and atherothrombosis. Semin Thromb Hemost. 2007;33:123–7. - PubMed
-
- Siegel-Axel DI, Gawaz M. Platelets and endothelial cells. Semin Thromb Hemost. 2007;33:128–35. - PubMed
-
- van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol. 2009;85:195–204. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

