Neuraminidase-deficient Sendai virus HN mutants provide protection from homologous superinfection
- PMID: 20024589
- PMCID: PMC2815292
- DOI: 10.1007/s00705-009-0567-6
Neuraminidase-deficient Sendai virus HN mutants provide protection from homologous superinfection
Abstract
Binding of hemagglutinin-neuraminidase proteins (HN) to sialylated receptors initiates the infection process of several paramyxoviruses, whereas later in the viral life cycle, the neuramindase (NA) activity of newly synthesized HN destroys all receptors. Prior to NA action, expressed HN has to bind the receptor. To evaluate this HN-receptor complex with respect to receptor inactivation, three temperature-sensitive Sendai virus HN mutants carrying amino acid exchanges at positions 262, 264 and/or 461 were created that uncoupled NA activity from receptor binding at 39 degrees Celsius. Interestingly, at elevated temperature, when there is no detectable neuramindase activity, all infected cells are protected against homologous superinfection. Mutated HN protein on the cell surface is mainly bound to sialylated cell-surface components but can be released by treatment with NA. Thus, continuous binding to HN already inactivates the receptors quantitatively. Furthermore, mutant HN bound to receptors is prevented from being incorporated into virus particles in the absence of NA. It is shown here for the first time that during paramyxoviral infection, quantitative receptor inactivation already occurs due to binding of receptors to expressed HN protein without involvement of NA and is independent of NA activity of viral progeny. NA subsequently functions in the release of HN from the complex, coupled with desialysation of receptors. These findings could have implications for further antiviral drug development.
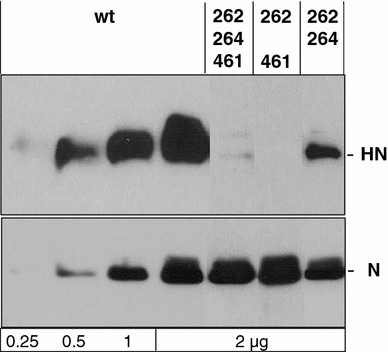
Figures

Similar articles
-
[Study on functions of N-carbohydrate chains in human parainfluenza virus type 3 hemagglutinin-neuraminidase protein].Bing Du Xue Bao. 2013 Sep;29(5):500-8. Bing Du Xue Bao. 2013. PMID: 24386838 Chinese.
-
Structural and functional relationship between the receptor recognition and neuraminidase activities of the Newcastle disease virus hemagglutinin-neuraminidase protein: receptor recognition is dependent on neuraminidase activity.J Virol. 2001 Feb;75(4):1918-27. doi: 10.1128/JVI.75.4.1918-1927.2001. J Virol. 2001. PMID: 11160691 Free PMC article.
-
Newcastle Disease Virus Establishes Persistent Infection in Tumor Cells In Vitro: Contribution of the Cleavage Site of Fusion Protein and Second Sialic Acid Binding Site of Hemagglutinin-Neuraminidase.J Virol. 2017 Jul 27;91(16):e00770-17. doi: 10.1128/JVI.00770-17. Print 2017 Aug 15. J Virol. 2017. PMID: 28592535 Free PMC article.
-
Recent developments with live-attenuated recombinant paramyxovirus vaccines.Rev Med Virol. 2013 Jan;23(1):15-34. doi: 10.1002/rmv.1717. Epub 2012 May 8. Rev Med Virol. 2013. PMID: 22570186 Review.
-
Sendai virus as a backbone for vaccines against RSV and other human paramyxoviruses.Expert Rev Vaccines. 2016;15(2):189-200. doi: 10.1586/14760584.2016.1114418. Epub 2015 Dec 9. Expert Rev Vaccines. 2016. PMID: 26648515 Free PMC article. Review.
Cited by
-
Recombinant Sendai Virus Vectors as Novel Vaccine Candidates Against Animal Viruses.Viruses. 2025 May 21;17(5):737. doi: 10.3390/v17050737. Viruses. 2025. PMID: 40431748 Free PMC article. Review.
-
Sendai virus intra-host population dynamics and host immunocompetence influence viral virulence during in vivo passage.Virus Evol. 2016 Apr 9;2(1):vew008. doi: 10.1093/ve/vew008. eCollection 2016 Jan. Virus Evol. 2016. PMID: 27774301 Free PMC article.
-
Evidence that Receptor Destruction by the Sendai Virus Hemagglutinin-Neuraminidase Protein Is Responsible for Homologous Interference.J Virol. 2016 Aug 12;90(17):7640-6. doi: 10.1128/JVI.01087-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27279623 Free PMC article.
References
-
- Alymova IV, Taylor G, Takimoto T, Lin T-H, Chand P, Babu YS, Li C, Xiong X, Portner A. Efficacy of novel hemagglutinin-neuraminidase inhibitors BCX 2798 and BCX 2855 against human parainfluenza viruses in vitro and in vivo. Antimicrob Agents Chemother. 2004;48:1495–1502. doi: 10.1128/AAC.48.5.1495-1502.2004. - DOI - PMC - PubMed
-
- Bitzer M, Ungerechts G, Bossow S, Graepler F, Sedlmeier R, Armeanu S, Bernloehr C, Spiegel M, Gross CD, Gregor M, Neubert WJ, Lauer UM. Negative-strand RNA viral vectors: intravenous application of Sendai virus vectors for the systemic delivery of therapeutic genes. Mol Ther. 2003;7:210–217. doi: 10.1016/S1525-0016(02)00052-7. - DOI - PubMed
-
- Connaris H, Takimoto T, Russell R, Crennell S, Moustafa I, Portner A, Taylor G. Probing the sialic acid binding site of the hemagglutinin-neuraminidase of Newcastle disease virus: identification of key amino acids involved in cell binding, catalysis, and fusion. J Virol. 2002;76:1816–1824. doi: 10.1128/JVI.76.4.1816-1824.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

