Mechanism of diepoxybutane-induced p53 regulation in human cells
- PMID: 20024960
- PMCID: PMC12089700
- DOI: 10.1002/jbt.20300
Mechanism of diepoxybutane-induced p53 regulation in human cells
Abstract
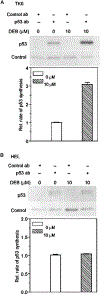
Diepoxybutane (DEB) is the most potent active metabolite of the environmental chemical 1,3-butadiene (BD). BD is a known mutagen and human carcinogen and possesses multisystems organ toxicity. We previously reported the elevation of p53 in human TK6 lymphoblasts undergoing DEB-induced apoptosis. In this study, we have characterized the DEB-induced p53 accumulation and investigated the mechanisms by which DEB regulates this p53 accumulation. The elevation of p53 levels in DEB-exposed TK6 lymphoblasts and human embryonic lung (HEL) human fibroblasts was found to be largely due to the stabilization of the p53 protein. DEB increased the acetylation of p53 at lys-382, dramatically reduced complex formation between p53 and its regulator protein mdm2 and induced the phosphorylation of p53 at serines 15, 20, 37, 46, and 392 in human lymphoblasts. A dramatic increase in phosphorylation of p53 at serine 15 in correlation to total p53 levels was observed in DEB-exposed Ataxia Telangiectasia Mutated (ATM) proficient human lymphoblasts as compared to DEB-exposed ATM-deficient human lymphoblasts; this implicates the ATM kinase in the elevation of p53 levels in DEB-exposed cells. Collectively, these findings explain for the first time the mechanism by which p53 accumulates in DEB-exposed cells and contributes to the understanding of the molecular toxicity of DEB and BD.
Figures






Similar articles
-
Diepoxybutane induces caspase and p53-mediated apoptosis in human lymphoblasts.Toxicol Appl Pharmacol. 2004 Mar 1;195(2):154-65. doi: 10.1016/j.taap.2003.11.006. Toxicol Appl Pharmacol. 2004. PMID: 14998682
-
Diepoxybutane induces the expression of a novel p53-target gene XCL1 that mediates apoptosis in exposed human lymphoblasts.J Biochem Mol Toxicol. 2020 Mar;34(3):e22446. doi: 10.1002/jbt.22446. Epub 2020 Jan 18. J Biochem Mol Toxicol. 2020. PMID: 31953984 Free PMC article.
-
Diepoxybutane induces the p53-dependent transactivation of the CCL4 gene that mediates apoptosis in exposed human lymphoblasts.J Biochem Mol Toxicol. 2023 May;37(5):e23316. doi: 10.1002/jbt.23316. Epub 2023 Feb 12. J Biochem Mol Toxicol. 2023. PMID: 36775894 Free PMC article.
-
How to activate p53.Curr Biol. 2000 Apr 20;10(8):R315-7. doi: 10.1016/s0960-9822(00)00439-5. Curr Biol. 2000. PMID: 10801407 Review.
-
[P53-independent apoptosis through a novel ATM/IKK-alpha/p73-mediated apoptotic pathway].Seikagaku. 2008 May;80(5):409-13. Seikagaku. 2008. PMID: 18575226 Review. Japanese. No abstract available.
Cited by
-
Diepoxybutane-induced apoptosis is mediated through the ERK1/2 pathway.Hum Exp Toxicol. 2018 Oct;37(10):1080-1091. doi: 10.1177/0960327118755255. Epub 2018 Feb 6. Hum Exp Toxicol. 2018. PMID: 29405768 Free PMC article.
-
Mechanisms of environmental chemicals that enable the cancer hallmark of evasion of growth suppression.Carcinogenesis. 2015 Jun;36 Suppl 1(Suppl 1):S2-18. doi: 10.1093/carcin/bgv028. Carcinogenesis. 2015. PMID: 26106139 Free PMC article. Review.
References
-
- National Toxicology Program. Ninth Report on Carcinogens. Research Triangle Park, NC: U.S. Department of Health and Human Services; 2000.
-
- U.S. Environmental Protection Agency. Health Assessment of 1,3-Butadiene: Office of Research and Development, National Center for Environmental Assessment; 2002. 66151–66152.
-
- Doerr JK, Hollis EA, Sipes IG. Species difference in the ovarian toxicity of 1,3-butadiene epoxides in B6C3F1 mice and Sprague–Dawley rats. Toxicology 1996;113(1–3):128–136. - PubMed
-
- Melnick RL, Sills RC. Comparative carcinogenicity of 1,3-butadiene, isoprene, and chloroprene in rats and mice. Chem Biol Interact 2001;135–136:27–42. - PubMed
-
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of 1,3-Butadiene (CAS No. 106–99-0) in B6C3F1 Mice (Inhalation Studies): National Toxicol. Program Tech. Rep. Ser; 1993 May 1993. 1–389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

