Changes in afferent activity after spinal cord injury
- PMID: 20025033
- PMCID: PMC2891065
- DOI: 10.1002/nau.20761
Changes in afferent activity after spinal cord injury
Abstract
Aims: To summarize the changes that occur in the properties of bladder afferent neurons following spinal cord injury.
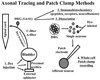
Methods: Literature review of anatomical, immunohistochemical, and pharmacologic studies of normal and dysfunctional bladder afferent pathways.
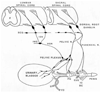
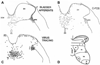
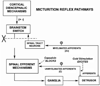
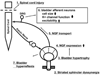
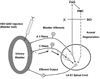
Results: Studies in animals indicate that the micturition reflex is mediated by a spinobulbospinal pathway passing through coordination centers (periaqueductal gray and pontine micturition center) located in the rostral brain stem. This reflex pathway, which is activated by small myelinated (Adelta) bladder afferent nerves, is in turn modulated by higher centers in the cerebral cortex involved in the voluntary control of micturition. Spinal cord injury at cervical or thoracic levels disrupts voluntary voiding, as well as the normal reflex pathways that coordinate bladder and sphincter function. Following spinal cord injury, the bladder is initially areflexic but then becomes hyperreflexic due to the emergence of a spinal micturition reflex pathway. The recovery of bladder function after spinal cord injury is dependent in part on the plasticity of bladder afferent pathways and the unmasking of reflexes triggered by unmyelinated, capsaicin-sensitive, C-fiber bladder afferent neurons. Plasticity is associated with morphologic, chemical, and electrical changes in bladder afferent neurons and appears to be mediated in part by neurotrophic factors released in the spinal cord and the peripheral target organs.
Conclusions: Spinal cord injury at sites remote from the lumbosacral spinal cord can indirectly influence properties of bladder afferent neurons by altering the function and chemical environment in the bladder or the spinal cord.
Conflict of interest statement
Conflicts of interest: none.
Figures




Similar articles
-
Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury.Prog Brain Res. 2006;152:59-84. doi: 10.1016/S0079-6123(05)52005-3. Prog Brain Res. 2006. PMID: 16198694 Review.
-
Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury.Prog Brain Res. 2006;152:97-115. doi: 10.1016/S0079-6123(05)52007-7. Prog Brain Res. 2006. PMID: 16198696 Review.
-
Plasticity in reflex pathways to the lower urinary tract following spinal cord injury.Exp Neurol. 2012 May;235(1):123-32. doi: 10.1016/j.expneurol.2011.05.003. Epub 2011 May 9. Exp Neurol. 2012. PMID: 21596038 Free PMC article. Review.
-
Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury.Exp Neurol. 2005 Mar;192(1):46-59. doi: 10.1016/j.expneurol.2004.10.017. Exp Neurol. 2005. PMID: 15698618
-
Developmental and injury induced plasticity in the micturition reflex pathway.Behav Brain Res. 1998 May;92(2):127-40. doi: 10.1016/s0166-4328(97)00185-x. Behav Brain Res. 1998. PMID: 9638955 Review.
Cited by
-
Renal function in a rat model of neurogenic bladder, effect of statins and phosphodiesterase-5 inhibitors.Eur Spine J. 2013 Dec;22(12):2766-9. doi: 10.1007/s00586-013-2927-x. Epub 2013 Aug 2. Eur Spine J. 2013. PMID: 23903999 Free PMC article.
-
Non-invasive Neuromodulation of Spinal Cord Restores Lower Urinary Tract Function After Paralysis.Front Neurosci. 2018 Jun 29;12:432. doi: 10.3389/fnins.2018.00432. eCollection 2018. Front Neurosci. 2018. PMID: 30008661 Free PMC article.
-
Propriospinal Neurons of L3-L4 Segments Involved in Control of the Rat External Urethral Sphincter.Neuroscience. 2020 Jan 15;425:12-28. doi: 10.1016/j.neuroscience.2019.11.013. Epub 2019 Nov 27. Neuroscience. 2020. PMID: 31785359 Free PMC article.
-
An animal study to compare the degree of the suppressive effects on the afferent pathways of micturition between tamsulosin and sildenafil.J Biomed Sci. 2013 Oct 25;20(1):81. doi: 10.1186/1423-0127-20-81. J Biomed Sci. 2013. PMID: 24160992 Free PMC article. Clinical Trial.
-
Effects of lateral funiculus sparing, spinal lesion level, and gender on recovery of bladder voiding reflexes and hematuria in rats.J Neurotrauma. 2015 Feb 1;32(3):200-8. doi: 10.1089/neu.2013.3247. Epub 2014 Dec 10. J Neurotrauma. 2015. PMID: 25137571 Free PMC article.
References
-
- Barrington FJF. The effect of lesion of the hind-and mid brain on micturition in the cat. Q J Exp Physiol. 1925;15:81–102.
-
- Chancellor MB, Yoshimura N. Physiology and pharmacology of the bladder and urethra. In: Wein AJ, editor. Campbell-walsh urology. 9th edition. Philadelphia, PA, USA: B. Saunders Elserion; 2006. pp. 1922–1972. Chapter 56.
-
- de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. Nervous control of the urogenital system. London, UK: Harwood Academic Publishers; 1993. pp. 227–290.
-
- Everaerts W, Gevaert T, Nilius B, et al. On the origin of bladder sensing: Tr(i)ps in urology. Neurourol Urodyn. 2008;27:264–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

