High-frequency stimulation of the subthalamic nucleus restores neural and behavioral functions during reaction time task in a rat model of Parkinson's disease
- PMID: 20025062
- PMCID: PMC4387000
- DOI: 10.1002/jnr.22313
High-frequency stimulation of the subthalamic nucleus restores neural and behavioral functions during reaction time task in a rat model of Parkinson's disease
Abstract
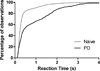
Deep brain stimulation (DBS) has been used in the clinic to treat Parkinson's disease (PD) and other neuropsychiatric disorders. Our previous work has shown that DBS in the subthalamic nucleus (STN) can improve major motor deficits, and induce a variety of neural responses in rats with unilateral dopamine (DA) lesions. In the present study, we examined the effect of STN DBS on reaction time (RT) performance and parallel changes in neural activity in the cortico-basal ganglia regions of partially bilateral DA- lesioned rats. We recorded neural activity with a multiple-channel single-unit electrode system in the primary motor cortex (MI), the STN, and the substantia nigra pars reticulata (SNr) during RT test. RT performance was severely impaired following bilateral injection of 6-OHDA into the dorsolateral part of the striatum. In parallel with such behavioral impairments, the number of responsive neurons to different behavioral events was remarkably decreased after DA lesion. Bilateral STN DBS improved RT performance in 6-OHDA lesioned rats, and restored operational behavior-related neural responses in cortico-basal ganglia regions. These behavioral and electrophysiological effects of DBS lasted nearly an hour after DBS termination. These results demonstrate that a partial DA lesion-induced impairment of RT performance is associated with changes in neural activity in the cortico-basal ganglia circuit. Furthermore, STN DBS can reverse changes in behavior and neural activity caused by partial DA depletion. The observed long-lasting beneficial effect of STN DBS suggests the involvement of the mechanism of neural plasticity in modulating cortico-basal ganglia circuits.
(c) 2009 Wiley-Liss, Inc.
Figures





Similar articles
-
Basal ganglia neural responses during behaviorally effective deep brain stimulation of the subthalamic nucleus in rats performing a treadmill locomotion test.Synapse. 2006 Jun 1;59(7):445-57. doi: 10.1002/syn.20261. Synapse. 2006. PMID: 16521122
-
Subthalamic nucleus stimulation increases brain derived neurotrophic factor in the nigrostriatal system and primary motor cortex.J Parkinsons Dis. 2011;1(1):123-36. J Parkinsons Dis. 2011. PMID: 22328911 Free PMC article.
-
Deep brain stimulation of the subthalamic nucleus in the 6-hydroxydopamine rat model of Parkinson's disease: effects on sensorimotor gating.Behav Brain Res. 2012 Apr 21;230(1):243-50. doi: 10.1016/j.bbr.2012.02.009. Epub 2012 Feb 11. Behav Brain Res. 2012. PMID: 22330948
-
Subthalamic nucleus deep brain stimulation on motor-symptoms of Parkinson's disease: Focus on neurochemistry.Prog Neurobiol. 2017 Apr;151:157-174. doi: 10.1016/j.pneurobio.2017.01.003. Epub 2017 Jan 31. Prog Neurobiol. 2017. PMID: 28159574 Review.
-
An electrophysiological perspective on Parkinson's disease: symptomatic pathogenesis and therapeutic approaches.J Biomed Sci. 2021 Dec 9;28(1):85. doi: 10.1186/s12929-021-00781-z. J Biomed Sci. 2021. PMID: 34886870 Free PMC article. Review.
Cited by
-
Effects of subthalamic deep brain stimulation on striatal metabolic connectivity in a rat hemiparkinsonian model.Dis Model Mech. 2019 May 24;12(5):dmm039065. doi: 10.1242/dmm.039065. Dis Model Mech. 2019. PMID: 31064773 Free PMC article.
-
High-Frequency Stimulation of the Rat Entopeduncular Nucleus Does Not Provide Functional or Morphological Neuroprotection from 6-Hydroxydopamine.PLoS One. 2015 Jul 29;10(7):e0133957. doi: 10.1371/journal.pone.0133957. eCollection 2015. PLoS One. 2015. PMID: 26222442 Free PMC article.
-
Deep Brain Stimulation Frequency of the Subthalamic Nucleus Affects Phonemic and Action Fluency in Parkinson's Disease.Parkinsons Dis. 2016;2016:6760243. doi: 10.1155/2016/6760243. Epub 2016 Dec 5. Parkinsons Dis. 2016. PMID: 28050309 Free PMC article.
-
A Novel Approach to Assess Motor Outcome of Deep Brain Stimulation Effects in the Hemiparkinsonian Rat: Staircase and Cylinder Test.J Vis Exp. 2016 May 31;(111):53951. doi: 10.3791/53951. J Vis Exp. 2016. PMID: 27284739 Free PMC article.
-
Reduction of Continuous Theta Burst Stimulation-Induced Motor Plasticity in Healthy Elderly With COMT Val158Met Polymorphism.Ann Rehabil Med. 2014 Oct;38(5):658-64. doi: 10.5535/arm.2014.38.5.658. Epub 2014 Oct 30. Ann Rehabil Med. 2014. PMID: 25379495 Free PMC article.
References
-
- Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Movement Disord. 2003;18:231–240. - PubMed
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. - PubMed
-
- Amalric M, Koob GF. Dorsal pallidum as a functional motor output of the corpus striatum. Brain Res. 1989;483:389–394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

