Mass spectral analysis of neuropeptide expression and distribution in the nervous system of the lobster Homarus americanus
- PMID: 20025296
- PMCID: PMC2833335
- DOI: 10.1021/pr900736t
Mass spectral analysis of neuropeptide expression and distribution in the nervous system of the lobster Homarus americanus
Abstract
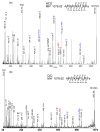
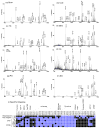
The lobster Homarus americanus has long served as an important animal model for electrophysiological and behavioral studies. Using this model, we performed a comprehensive investigation of the neuropeptide expression and their localization in the nervous system, which provides useful insights for further understanding of their biological functions. Using nanoLC ESI Q-TOF MS/MS and three types of MALDI instruments, we analyzed the neuropeptide complements in a major neuroendocrine structure, pericardial organ. A total of 57 putative neuropeptides were identified and 18 of them were de novo sequenced. Using direct tissue/extract analysis and bioinformatics software SpecPlot, we charted the global distribution of neuropeptides throughout the nervous system in H. americanus. Furthermore, we also mapped the localization of several neuropeptide families in the brain by high mass resolution and high mass accuracy mass spectrometric imaging (MSI) using a MALDI LTQ Orbitrap mass spectrometer. We have also compared the utility and instrument performance of multiple mass spectrometers for neuropeptide analysis in terms of peptidome coverage, sensitivity, mass spectral resolution and capability for de novo sequencing.
Figures





Similar articles
-
Mass spectral characterization of peptide transmitters/hormones in the nervous system and neuroendocrine organs of the American lobster Homarus americanus.Gen Comp Endocrinol. 2008 Apr 1;156(2):395-409. doi: 10.1016/j.ygcen.2008.01.009. Epub 2008 Jan 26. Gen Comp Endocrinol. 2008. PMID: 18304551 Free PMC article.
-
Defining the Neuropeptidome of the Spiny Lobster Panulirus interruptus Brain Using a Multidimensional Mass Spectrometry-Based Platform.J Proteome Res. 2015 Nov 6;14(11):4776-91. doi: 10.1021/acs.jproteome.5b00627. Epub 2015 Oct 5. J Proteome Res. 2015. PMID: 26390183 Free PMC article.
-
C-terminal methylation of truncated neuropeptides: an enzyme-assisted extraction artifact involving methanol.Peptides. 2013 Aug;46:108-25. doi: 10.1016/j.peptides.2013.05.008. Epub 2013 May 25. Peptides. 2013. PMID: 23714174
-
Neuropeptidomics: Improvements in Mass Spectrometry Imaging Analysis and Recent Advancements.Curr Protein Pept Sci. 2021;22(2):158-169. doi: 10.2174/1389203721666201116115708. Curr Protein Pept Sci. 2021. PMID: 33200705 Free PMC article. Review.
-
Discovering new invertebrate neuropeptides using mass spectrometry.Mass Spectrom Rev. 2006 Jan-Feb;25(1):77-98. doi: 10.1002/mas.20055. Mass Spectrom Rev. 2006. PMID: 15937922 Review.
Cited by
-
Mapping of neuropeptides in the crustacean stomatogastric nervous system by imaging mass spectrometry.J Am Soc Mass Spectrom. 2013 Jan;24(1):134-47. doi: 10.1007/s13361-012-0502-z. Epub 2012 Nov 29. J Am Soc Mass Spectrom. 2013. PMID: 23192703 Free PMC article.
-
Automated querying and identification of novel peptides using MALDI mass spectrometric imaging.J Proteome Res. 2011 Apr 1;10(4):1915-28. doi: 10.1021/pr101159e. Epub 2011 Mar 15. J Proteome Res. 2011. PMID: 21332220 Free PMC article.
-
In situ identification and mapping of neuropeptides from the stomatogastric nervous system of Cancer borealis.Rapid Commun Mass Spectrom. 2014 Nov 30;28(22):2437-44. doi: 10.1002/rcm.7037. Rapid Commun Mass Spectrom. 2014. PMID: 25303472 Free PMC article.
-
Mass spectrometric analysis of spatio-temporal dynamics of crustacean neuropeptides.Biochim Biophys Acta. 2015 Jul;1854(7):798-811. doi: 10.1016/j.bbapap.2014.10.023. Epub 2014 Nov 4. Biochim Biophys Acta. 2015. PMID: 25448012 Free PMC article. Review.
-
MALDI mass spectrometry imaging of neuronal cell cultures.J Am Soc Mass Spectrom. 2011 May;22(5):828-36. doi: 10.1007/s13361-011-0111-2. Epub 2011 Mar 26. J Am Soc Mass Spectrom. 2011. PMID: 21472517 Free PMC article.
References
-
- Bucher D, Taylor AL, Marder E. Central pattern generating neurons simultaneously express fast and slow rhythmic activities in the stomatogastric ganglion. J Neurophysiol. 2006;95(6):3617–3632. - PubMed
-
- Beltz B, Eisen JS, Flamm R, Harris-Warrick RM, Hooper SL, Marder E. Serotonergic innervation and modulation of the stomatogastric ganglion of three decapod crustaceans (Panulirus interruptus, Homarus americanus and Cancer irroratus) J Exp Biol. 1984;109(1):35–54. - PubMed
-
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Ann Rev Physiol. 2007;69(1):291–316. - PubMed
-
- Bolingbroke M, Kass-Simon G. 20-Hydroxyecdysone Causes Increased Aggressiveness in Female American Lobsters, Homarus americanus. Horm Behav. 2001;39(2):144–156. - PubMed
-
- Cromarty SI, Mello J, Kass-Simon G. Molt-related and size-dependent differences in the escape response and post-threat behavior of the American lobster, Homarus americanus. Biol Bull. 2000;199(3):265–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

