Direct MinE-membrane interaction contributes to the proper localization of MinDE in E. coli
- PMID: 20025670
- PMCID: PMC2814086
- DOI: 10.1111/j.1365-2958.2009.07006.x
Direct MinE-membrane interaction contributes to the proper localization of MinDE in E. coli
Abstract
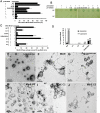
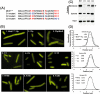
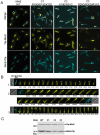
Dynamic oscillation of the Min system in Escherichia coli determines the placement of the division plane at the midcell. In addition to stimulating MinD ATPase activity, we report here that MinE can directly interact with the membrane and this interaction contributes to the proper MinDE localization and dynamics. The N-terminal domain of MinE is involved in direct contact between MinE and the membranes that may subsequently be stabilized by the C-terminal domain of MinE. In an in vitro system, MinE caused liposome deformation into membrane tubules, a property similar to that previously reported for MinD. We isolated a mutant MinE containing residue substitutions in R10, K11 and K12 that was fully capable of stimulating MinD ATPase activity, but was deficient in membrane binding. Importantly, this mutant was unable to support normal MinDE localization and oscillation, suggesting that direct MinE interaction with the membrane is critical for the dynamic behavior of the Min system.
Figures


Similar articles
-
Mapping the MinE site involved in interaction with the MinD division site selection protein of Escherichia coli.J Bacteriol. 2003 Aug;185(16):4948-55. doi: 10.1128/JB.185.16.4948-4955.2003. J Bacteriol. 2003. PMID: 12897015 Free PMC article.
-
Min protein patterns emerge from rapid rebinding and membrane interaction of MinE.Nat Struct Mol Biol. 2011 May;18(5):577-83. doi: 10.1038/nsmb.2037. Epub 2011 Apr 24. Nat Struct Mol Biol. 2011. PMID: 21516096
-
A multistranded polymer model explains MinDE dynamics in E. coli cell division.Biophys J. 2007 Aug 15;93(4):1134-50. doi: 10.1529/biophysj.106.097162. Epub 2007 May 4. Biophys J. 2007. PMID: 17483175 Free PMC article.
-
Spatial control of the cell division site by the Min system in Escherichia coli.Environ Microbiol. 2013 Dec;15(12):3229-39. doi: 10.1111/1462-2920.12119. Epub 2013 Apr 9. Environ Microbiol. 2013. PMID: 23574354 Review.
-
MinD and role of the deviant Walker A motif, dimerization and membrane binding in oscillation.Mol Microbiol. 2003 Apr;48(2):295-303. doi: 10.1046/j.1365-2958.2003.03427.x. Mol Microbiol. 2003. PMID: 12675792 Review.
Cited by
-
C-terminal eYFP fusion impairs Escherichia coli MinE function.Open Biol. 2020 May;10(5):200010. doi: 10.1098/rsob.200010. Epub 2020 May 27. Open Biol. 2020. PMID: 32456552 Free PMC article.
-
An Optimal Free Energy Dissipation Strategy of the MinCDE Oscillator in Regulating Symmetric Bacterial Cell Division.PLoS Comput Biol. 2015 Aug 28;11(8):e1004351. doi: 10.1371/journal.pcbi.1004351. eCollection 2015 Aug. PLoS Comput Biol. 2015. PMID: 26317492 Free PMC article.
-
Cardiolipin Synthesis and Outer Membrane Localization Are Required for Shigella flexneri Virulence.mBio. 2017 Aug 29;8(4):e01199-17. doi: 10.1128/mBio.01199-17. mBio. 2017. PMID: 28851846 Free PMC article.
-
Dissecting the role of conformational change and membrane binding by the bacterial cell division regulator MinE in the stimulation of MinD ATPase activity.J Biol Chem. 2017 Dec 15;292(50):20732-20743. doi: 10.1074/jbc.M117.805945. Epub 2017 Oct 24. J Biol Chem. 2017. PMID: 29066619 Free PMC article.
-
Symmetry and scale orient Min protein patterns in shaped bacterial sculptures.Nat Nanotechnol. 2015 Aug;10(8):719-26. doi: 10.1038/nnano.2015.126. Epub 2015 Jun 22. Nat Nanotechnol. 2015. PMID: 26098227 Free PMC article.
References
-
- de Boer PAJ, Crossley RE, Rothfield LI. A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum in E. coli. Cell. 1989;56:641–649. - PubMed
-
- Casadaban MJ, Cohen SN. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980;138:179–207. - PubMed
-
- Frottin F, Martinez A, Peynot P, Mitra S, Holz RC, Giglione C, Meinnel T. The proteomics of N-terminal methionine cleavage. Mol Cell Proteomics. 2006;5:2336–2349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

