Late treatment with imatinib mesylate ameliorates radiation-induced lung fibrosis in a mouse model
- PMID: 20025728
- PMCID: PMC2802357
- DOI: 10.1186/1748-717X-4-66
Late treatment with imatinib mesylate ameliorates radiation-induced lung fibrosis in a mouse model
Abstract
Background: We have previously shown that small molecule PDGF receptor tyrosine kinase inhibitors (RTKI) can drastically attenuate radiation-induced pulmonary fibrosis if the drug administration starts at the time of radiation during acute inflammation with present but limited effects against acute inflammation. To rule out interactions of the drug with acute inflammation, we investigated here in an interventive trial if a later drug administration start at a time when the acute inflammation has subsided--has also beneficial antifibrotic effects.
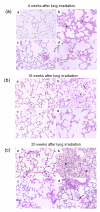
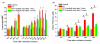
Methods: Whole thoraces of C57BL/6 mice were irradiated with 20 Gy and treated with the RTKI imatinib starting either 3 days after radiation (during acute inflammation) or two weeks after radiation (after the acute inflammation has subsided as demonstrated by leucocyte count). Lungs were monitored and analyzed by clinical, histological and in vivo non-invasive computed tomography as a quantitative measure for lung density and lung fibrosis.
Results: Irradiation induced severe lung fibrosis resulting in markedly reduced mouse survival vs. non-irradiated controls. Both early start of imatinib treatment during inflammation and late imatinib start markedly attenuated the development of pulmonary fibrosis as demonstrated by clinical, histological and qualitative and quantitative computed tomography results such as reduced lung density. Both administration schedules resulted in prolonged lifespans. The earlier drug treatment start resulted in slightly stronger beneficial antifibrotic effects along all measured endpoints than the later start.
Conclusions: Our findings show that imatinib, even when administered after the acute inflammation has subsided, attenuates radiation-induced lung fibrosis in mice. Our data also indicate that the fibrotic fate is not only determined by the early inflammatory events but rather a complex process in which secondary events at later time points are important. Because of the clinical availability of imatinib or similar compounds, a meaningful attenuation of radiation-induced lung fibrosis in patients seems possible.
Figures

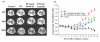
Similar articles
-
[Effect of imatinib mesylate on bleomycin-induced pulmonary fibrosis in mice].Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2007 Apr;19(4):229-32. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2007. PMID: 17448279 Chinese.
-
Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis.Arthritis Rheum. 2007 Jan;56(1):311-22. doi: 10.1002/art.22314. Arthritis Rheum. 2007. PMID: 17195235
-
Treatment with imatinib prevents fibrosis in different preclinical models of systemic sclerosis and induces regression of established fibrosis.Arthritis Rheum. 2009 Jan;60(1):219-24. doi: 10.1002/art.24186. Arthritis Rheum. 2009. PMID: 19116940
-
Tyrosine kinase inhibitors in the treatment of systemic sclerosis: from animal models to clinical trials.Curr Rheumatol Rep. 2011 Feb;13(1):21-7. doi: 10.1007/s11926-010-0142-x. Curr Rheumatol Rep. 2011. PMID: 21042889 Review.
-
Evaluation of imatinib mesylate in the treatment of pulmonary arterial hypertension.Future Cardiol. 2010 Jan;6(1):19-35. doi: 10.2217/fca.09.54. Future Cardiol. 2010. PMID: 20014985 Review.
Cited by
-
Radiation-Induced Lung Fibrosis: Preclinical Animal Models and Therapeutic Strategies.Cancers (Basel). 2020 Jun 12;12(6):1561. doi: 10.3390/cancers12061561. Cancers (Basel). 2020. PMID: 32545674 Free PMC article. Review.
-
Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis.Eur Respir J. 2015 May;45(5):1434-45. doi: 10.1183/09031936.00174914. Epub 2015 Mar 5. Eur Respir J. 2015. PMID: 25745043 Free PMC article. Review.
-
Quantitative assessment of radiation dose and fractionation effects on normal tissue by utilizing a novel lung fibrosis index model.Radiat Oncol. 2017 Nov 7;12(1):172. doi: 10.1186/s13014-017-0912-y. Radiat Oncol. 2017. PMID: 29116014 Free PMC article.
-
Challenges and Benefits of Repurposing Products for Use during a Radiation Public Health Emergency: Lessons Learned from Biological Threats and other Disease Treatments.Radiat Res. 2018 Dec;190(6):659-676. doi: 10.1667/RR15137.1. Epub 2018 Aug 30. Radiat Res. 2018. PMID: 30160600 Free PMC article.
-
Radiotherapy-induced heart disease: a review of the literature.Precis Clin Med. 2019 Dec;2(4):270-282. doi: 10.1093/pcmedi/pbz025. Epub 2019 Nov 29. Precis Clin Med. 2019. PMID: 35693876 Free PMC article. Review.
References
-
- Hurkmans CW, Cuijpers JP, Lagerwaard FJ, Widder J, Heide UA van der, Schuring D, Senan S. Recommendations for implementing stereotactic radiotherapy in peripheral stage IA non-small cell lung cancer: report from the Quality Assurance Working Party of the randomised phase III ROSEL study. Radiat Oncol. 2009;4:1. doi: 10.1186/1748-717X-4-1. - DOI - PMC - PubMed
-
- Uitterhoeve AL, Koolen MG, van Os RM, Koedooder K, Kar M van de, Pieters BR, Koning CC. Accelerated high-dose radiotherapy alone or combined with either concomitant or sequential chemotherapy; treatments of choice in patients with Non-Small Cell Lung Cancer. Radiat Oncol. 2007;2:27. doi: 10.1186/1748-717X-2-27. - DOI - PMC - PubMed
-
- Collins BT, Erickson K, Reichner CA, Collins SP, Gagnon GJ, Dieterich S, McRae DA, Zhang Y, Yousefi S, Levy E, Chang T, Jamis-Dow C, Banovac F, Anderson ED. Radical stereotactic radiosurgery with real-time tumor motion tracking in the treatment of small peripheral lung tumors. Radiat Oncol. 2007;2:39. doi: 10.1186/1748-717X-2-39. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

