Comparative proteomic analysis of pathogenic and non-pathogenic strains from the swine pathogen Mycoplasma hyopneumoniae
- PMID: 20025764
- PMCID: PMC2804596
- DOI: 10.1186/1477-5956-7-45
Comparative proteomic analysis of pathogenic and non-pathogenic strains from the swine pathogen Mycoplasma hyopneumoniae
Abstract
Background: Mycoplasma hyopneumoniae is a highly infectious swine pathogen and is the causative agent of enzootic pneumonia (EP). Following the previous report of a proteomic survey of the pathogenic 7448 strain of swine pathogen, Mycoplasma hyopneumoniae, we performed comparative protein profiling of three M. hyopneumoniae strains, namely the non-pathogenic J strain and the two pathogenic strains 7448 and 7422.
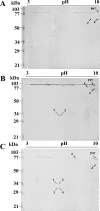
Results: In 2DE comparisons, we were able to identify differences in expression levels for 67 proteins, including the overexpression of some cytoadherence-related proteins only in the pathogenic strains. 2DE immunoblot analyses allowed the identification of differential proteolytic cleavage patterns of the P97 adhesin in the three strains. For more comprehensive protein profiling, an LC-MS/MS strategy was used. Overall, 35% of the M. hyopneumoniae genome coding capacity was covered. Partially overlapping profiles of identified proteins were observed in the strains with 81 proteins identified only in one strain and 54 proteins identified in two strains. Abundance analysis of proteins detected in more than one strain demonstrates the relative overexpression of 64 proteins, including the P97 adhesin in the pathogenic strains.
Conclusions: Our results indicate the physiological differences between the non-pathogenic strain, with its non-infective proliferate lifestyle, and the pathogenic strains, with its constitutive expression of adhesins, which would render the bacterium competent for adhesion and infection prior to host contact.
Figures


Similar articles
-
Differential secretome profiling of a swine tracheal cell line infected with mycoplasmas of the swine respiratory tract.J Proteomics. 2019 Feb 10;192:147-159. doi: 10.1016/j.jprot.2018.08.018. Epub 2018 Aug 31. J Proteomics. 2019. PMID: 30176387
-
Evidences of differential endoproteolytic processing on the surfaces of Mycoplasma hyopneumoniae and Mycoplasma flocculare.Microb Pathog. 2020 Mar;140:103958. doi: 10.1016/j.micpath.2019.103958. Epub 2019 Dec 30. Microb Pathog. 2020. PMID: 31899326
-
Proteomic survey of the pathogenic Mycoplasma hyopneumoniae strain 7448 and identification of novel post-translationally modified and antigenic proteins.Vet Microbiol. 2007 Mar 31;121(1-2):83-93. doi: 10.1016/j.vetmic.2006.11.018. Epub 2006 Nov 26. Vet Microbiol. 2007. PMID: 17182197
-
Pathogenicity & virulence of Mycoplasma hyopneumoniae.Virulence. 2020 Dec;11(1):1600-1622. doi: 10.1080/21505594.2020.1842659. Virulence. 2020. PMID: 33289597 Free PMC article. Review.
-
Mycoplasma hyopneumoniae variability: Current trends and proposed terminology for genomic classification.Transbound Emerg Dis. 2019 Sep;66(5):1840-1854. doi: 10.1111/tbed.13233. Epub 2019 Jun 4. Transbound Emerg Dis. 2019. PMID: 31099490 Review.
Cited by
-
Recombinant secreted antigens from Mycoplasma hyopneumoniae delivered as a cocktail vaccine enhance the immune response of mice.Clin Vaccine Immunol. 2013 Sep;20(9):1370-6. doi: 10.1128/CVI.00140-13. Epub 2013 Jun 26. Clin Vaccine Immunol. 2013. PMID: 23803903 Free PMC article.
-
New insights on the biology of swine respiratory tract mycoplasmas from a comparative genome analysis.BMC Genomics. 2013 Mar 14;14:175. doi: 10.1186/1471-2164-14-175. BMC Genomics. 2013. PMID: 23497205 Free PMC article.
-
Mycoplasmas-Host Interaction: Mechanisms of Inflammation and Association with Cellular Transformation.Microorganisms. 2020 Sep 4;8(9):1351. doi: 10.3390/microorganisms8091351. Microorganisms. 2020. PMID: 32899663 Free PMC article. Review.
-
Proteomic survey of the cestode Mesocestoides corti during the first 24 hours of strobilar development.Parasitol Res. 2011 Mar;108(3):645-56. doi: 10.1007/s00436-010-2109-2. Epub 2010 Oct 15. Parasitol Res. 2011. PMID: 20953630
-
NADH oxidase of Mycoplasma hyopneumoniae functions as a potential mediator of virulence.BMC Vet Res. 2022 Apr 2;18(1):126. doi: 10.1186/s12917-022-03230-7. BMC Vet Res. 2022. PMID: 35366872 Free PMC article.
References
-
- Maes D, Verdonck M, Deluyker H, de Kruif A. Enzootic pneumonia in pigs. Vet Q. 1996;18:104–109. - PubMed
-
- Vasconcelos AT, Ferreira HB, Bizarro CV, Bonatto SL, Carvalho MO, Pinto PM. Swine and poultry pathogens: the complete genome sequences of two strains of Mycoplasma hyopneumoniae and a strain of Mycoplasma synoviae. J Bacteriol. 2005;187:5568–5577. doi: 10.1128/JB.187.16.5568-5577.2005. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

