Developmental dynamics of cone photoreceptors in the eel
- PMID: 20025774
- PMCID: PMC2807862
- DOI: 10.1186/1471-213X-9-71
Developmental dynamics of cone photoreceptors in the eel
Abstract
Background: Many fish alter their expressed visual pigments during development. The number of retinal opsins expressed and their type is normally related to the environment in which they live. Eels are known to change the expression of their rod opsins as they mature, but might they also change the expression of their cone opsins?
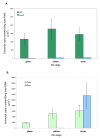
Results: The Rh2 and Sws2 opsin sequences from the European Eel were isolated, sequenced and expressed in vitro for an accurate measurement of their lambdamax values. In situ hybridisation revealed that glass eels express only rh2 opsin in their cone photoreceptors, while larger yellow eels continue to express rh2 opsin in the majority of their cones, but also have <5% of cones which express sws2 opsin. Silver eels showed the same expression pattern as the larger yellow eels. This observation was confirmed by qPCR (quantitative polymerase chain reaction).
Conclusions: Larger yellow and silver European eels express two different cone opsins, rh2 and sws2. This work demonstrates that only the Rh2 cone opsin is present in younger fish (smaller yellow and glass), the sws2 opsin being expressed additionally only by older fish and only in <5% of cone cells.
Figures




Similar articles
-
Spectral sensitivity of guppy visual pigments reconstituted in vitro to resolve association of opsins with cone cell types.Vision Res. 2016 Oct;127:67-73. doi: 10.1016/j.visres.2016.06.013. Epub 2016 Aug 3. Vision Res. 2016. PMID: 27476645
-
Molecular evidence that only two opsin subfamilies, the blue light- (SWS2) and green light-sensitive (RH2), drive color vision in Atlantic cod (Gadus morhua).PLoS One. 2014 Dec 31;9(12):e115436. doi: 10.1371/journal.pone.0115436. eCollection 2014. PLoS One. 2014. PMID: 25551396 Free PMC article.
-
Phylogenetic analysis and ontogenetic changes in the cone opsins of the western mosquitofish (Gambusia affinis).PLoS One. 2020 Oct 13;15(10):e0240313. doi: 10.1371/journal.pone.0240313. eCollection 2020. PLoS One. 2020. PMID: 33048954 Free PMC article.
-
Pronounced heritable variation and limited phenotypic plasticity in visual pigments and opsin expression of threespine stickleback photoreceptors.J Exp Biol. 2013 Feb 15;216(Pt 4):656-67. doi: 10.1242/jeb.078840. Epub 2012 Oct 17. J Exp Biol. 2013. PMID: 23077162
-
The visual cycle of the cone photoreceptors of the retina.Nutr Rev. 2004 Jul;62(7 Pt 1):283-6. doi: 10.1111/j.1753-4887.2004.tb00053.x. Nutr Rev. 2004. PMID: 15384919 Review.
Cited by
-
Variable light environments induce plastic spectral tuning by regional opsin coexpression in the African cichlid fish, Metriaclima zebra.Mol Ecol. 2015 Aug;24(16):4193-204. doi: 10.1111/mec.13312. Epub 2015 Aug 6. Mol Ecol. 2015. PMID: 26175094 Free PMC article.
-
Adult plasticity in African cichlids: Rapid changes in opsin expression in response to environmental light differences.Mol Ecol. 2017 Nov;26(21):6036-6052. doi: 10.1111/mec.14357. Epub 2017 Oct 9. Mol Ecol. 2017. PMID: 28926160 Free PMC article.
-
Developmental changes of opsin gene expression in ray-finned fishes (Actinopterygii).Proc Biol Sci. 2022 Nov 9;289(1986):20221855. doi: 10.1098/rspb.2022.1855. Epub 2022 Nov 2. Proc Biol Sci. 2022. PMID: 36321490 Free PMC article.
-
Gene expression patterns of novel visual and non-visual opsin families in immature and mature Japanese eel males.PeerJ. 2020 Feb 27;8:e8326. doi: 10.7717/peerj.8326. eCollection 2020. PeerJ. 2020. PMID: 32149019 Free PMC article.
-
Genomic insights into the seawater adaptation in Cyprinidae.BMC Biol. 2024 Apr 19;22(1):87. doi: 10.1186/s12915-024-01885-2. BMC Biol. 2024. PMID: 38637780 Free PMC article.
References
-
- Berry L, Brookes D, Walker B. The problem of the migration of the European eel (Anguilla anguilla) Science Progress. 1972;60:465–485.
-
- Tesch FW. The eel: biology and management of anguillid eels. London: Chapman and Hall; 1977.
-
- van Ginneken VJT, Maes GE. The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: a literature review. Rev Fish Biol Fisheries. 2005;15:367–398. doi: 10.1007/s11160-006-0005-8. - DOI
-
- Bowmaker JK, Loew ER. In: The Senses: a Comprehensive Reference 1 Vision. Masland RH, Albright TD, editor. Oxford: Elsevier; 2008. Vision in Fish; pp. 53–76. full_text.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

