Genetic fate mapping demonstrates contribution of epicardium-derived cells to the annulus fibrosis of the mammalian heart
- PMID: 20025864
- PMCID: PMC2815244
- DOI: 10.1016/j.ydbio.2009.12.007
Genetic fate mapping demonstrates contribution of epicardium-derived cells to the annulus fibrosis of the mammalian heart
Abstract
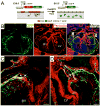
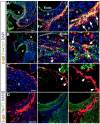
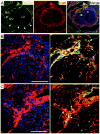
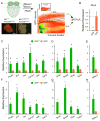
The annulus fibrosis electrically insulates the atria and ventricles, allowing the timed sequential beating of these structures that is necessary for efficient heart function. Abnormal development of the annulus fibrosis leads to persistence of accessory electrical pathways from atria to ventricles, providing the anatomical substrate for re-entrant cardiac arrhythmias such as Wolff-Parkinson-White syndrome. To better understand the development of the annulus fibrosis and the etiology of these cardiac arrhythmias, we used Cre-LoxP technology to assess the contribution of epicardium derived cells (EPDCs) to the annulus fibrosis. We found that EPDCs migrated into the region of the forming annulus fibrosis, marked by the protein periostin. These EPDCs also stained positive for procollagen I, suggesting that the EPDCs themselves synthesize proteins of the annulus fibrosis. To further test the hypothesis that EPDCs contribute to cells that synthesize the annulus fibrosis, we purified genetically marked EPDCs from the atrioventricular region and measured gene expression by quantitative PCR. These EPDCs were highly enriched for mRNAs encoding periostin, procollagen I, fibronectin I, vimentin, discoidin domain receptor 2, and tenascin C, markers of fibroblasts and components of the annulus fibrosis. In addition, these EPDCs were highly enriched for Snail, Smad1, Slug, and Twist1, markers for epithelial-to-mesenchymal transition (EMT), and a metalloprotease, Mmp2, that contributes to cellular migration. Our work provides for the first time definitive evidence that epicardium contributes to formation of the mammalian annulus fibrosis through EMT. Abnormalities of this differentiation process may underlie development of some forms of re-entrant atrioventricular tachycardia.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Alk3 mediated Bmp signaling controls the contribution of epicardially derived cells to the tissues of the atrioventricular junction.Dev Biol. 2014 Dec 1;396(1):8-18. doi: 10.1016/j.ydbio.2014.09.031. Epub 2014 Oct 6. Dev Biol. 2014. PMID: 25300579 Free PMC article.
-
Epicardium-derived cells in development of annulus fibrosis and persistence of accessory pathways.Circulation. 2008 Mar 25;117(12):1508-17. doi: 10.1161/CIRCULATIONAHA.107.726315. Epub 2008 Mar 10. Circulation. 2008. PMID: 18332266
-
Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart.Dev Biol. 2012 Jun 15;366(2):111-24. doi: 10.1016/j.ydbio.2012.04.020. Epub 2012 Apr 24. Dev Biol. 2012. PMID: 22546693 Free PMC article.
-
The arterial and cardiac epicardium in development, disease and repair.Differentiation. 2012 Jul;84(1):41-53. doi: 10.1016/j.diff.2012.05.002. Epub 2012 May 30. Differentiation. 2012. PMID: 22652098 Review.
-
Origin, fate, and function of epicardium-derived cells (EPDCs) in normal and abnormal cardiac development.ScientificWorldJournal. 2007 Nov 12;7:1777-98. doi: 10.1100/tsw.2007.294. ScientificWorldJournal. 2007. PMID: 18040540 Free PMC article. Review.
Cited by
-
Canonical wnt signaling regulates atrioventricular junction programming and electrophysiological properties.Circ Res. 2015 Jan 30;116(3):398-406. doi: 10.1161/CIRCRESAHA.116.304731. Epub 2014 Nov 6. Circ Res. 2015. PMID: 25599332 Free PMC article.
-
Interleukin-36 Cytokine/Receptor Signaling: A New Target for Tissue Fibrosis.Int J Mol Sci. 2020 Sep 4;21(18):6458. doi: 10.3390/ijms21186458. Int J Mol Sci. 2020. PMID: 32899668 Free PMC article. Review.
-
Platelet-derived growth factor receptor-α is essential for cardiac fibroblast survival.Am J Physiol Heart Circ Physiol. 2019 Aug 1;317(2):H330-H344. doi: 10.1152/ajpheart.00054.2019. Epub 2019 May 24. Am J Physiol Heart Circ Physiol. 2019. PMID: 31125253 Free PMC article.
-
Cardiac Fibroblast Diversity.Annu Rev Physiol. 2020 Feb 10;82:63-78. doi: 10.1146/annurev-physiol-021119-034527. Annu Rev Physiol. 2020. PMID: 32040933 Free PMC article. Review.
-
Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease.Circulation. 2012 Apr 10;125(14):1795-808. doi: 10.1161/CIRCULATIONAHA.111.040352. Circulation. 2012. PMID: 22492947 Free PMC article. Review. No abstract available.
References
-
- BECKER AE, ANDERSON RH, DURRER D, WELLENS HJ. The anatomical substrates of wolff-parkinson-white syndrome. A clinicopathologic correlation in seven patients. Circulation. 1978;57:870–879. - PubMed
-
- BUCKINGHAM M, MEILHAC S, ZAFFRAN S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. - PubMed
-
- FEIL R, WAGNER J, METZGER D, CHAMBON P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. - PubMed
-
- GITTENBERGER-DE GROOT AC, VRANCKEN PEETERS MP, MENTINK MM, GOURDIE RG, POELMANN RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 1998;82:1043–1052. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

