Polyol pathway impairs the function of SERCA and RyR in ischemic-reperfused rat hearts by increasing oxidative modifications of these proteins
- PMID: 20025885
- PMCID: PMC3043380
- DOI: 10.1016/j.yjmcc.2009.12.003
Polyol pathway impairs the function of SERCA and RyR in ischemic-reperfused rat hearts by increasing oxidative modifications of these proteins
Abstract
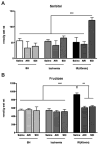
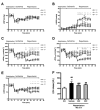
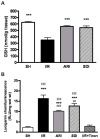
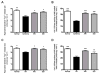
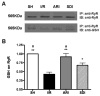
A number of studies have shown that the polyol pathway, consisting of aldose reductase (AR) and sorbitol dehydrogenase (SDH), contributes to ischemia-reperfusion (I/R)-induced myocardial infarction due to depletion of ATP. In this report we show that the polyol pathway in I/R heart also contributes to the impairment of sacro/endoplasmic reticulum Ca(2+)-ATPase (SERCA) and ryanodine receptor (RyR), two key players in Ca(2+) signaling that regulate cardiac contraction. Rat hearts were isolated and retrogradely perfused with either Krebs' buffer containing 1 microM AR inhibitor, zopolrestat, or 200 nM SDH inhibitor, CP-170,711, and challenged by 30 min of regional ischemia and 45 min of reperfusion. We found that post-ischemic contractile function of the isolated perfused hearts was improved by pharmacological inhibition of the polyol pathway. I/R-induced contractile dysfunction is most likely due to impairment in Ca(2+) signaling and the activities of SERCA and RyR. All these abnormalities were significantly ameliorated by treatment with ARI or SDI. We showed that the polyol pathway activities increase the level of peroxynitrite, which enhances the tyrosine nitration of SERCA and irreversibly modifies it to form SERCAC674-SO(3)H. This leads to reduced level of S-glutathiolated SERCA, contributing to its inactivation. The polyol pathway activities also deplete the level of GSH, leading to decreased active RyR, the S-glutathiolated RyR. Thus, in I/R heart, inhibition of polyol pathway improved the function of SERCA and RyR by protecting them from irreversible oxidation.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures

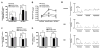
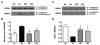

Similar articles
-
Cardiac contractile dysfunction during acute hyperglycemia due to impairment of SERCA by polyol pathway-mediated oxidative stress.Am J Physiol Cell Physiol. 2010 Sep;299(3):C643-53. doi: 10.1152/ajpcell.00137.2010. Epub 2010 Jun 23. Am J Physiol Cell Physiol. 2010. PMID: 20573996 Free PMC article.
-
Oxidation of ryanodine receptor after ischemia-reperfusion increases propensity of Ca2+ waves during β-adrenergic receptor stimulation.Am J Physiol Heart Circ Physiol. 2018 Oct 1;315(4):H1032-H1040. doi: 10.1152/ajpheart.00334.2018. Epub 2018 Jul 20. Am J Physiol Heart Circ Physiol. 2018. PMID: 30028204 Free PMC article.
-
Aldose reductase inhibition protects diabetic and nondiabetic rat hearts from ischemic injury.Diabetes. 1997 Feb;46(2):292-300. doi: 10.2337/diab.46.2.292. Diabetes. 1997. PMID: 9000707
-
Mitochondrial Bioenergetics During Ischemia and Reperfusion.Adv Exp Med Biol. 2017;982:141-167. doi: 10.1007/978-3-319-55330-6_8. Adv Exp Med Biol. 2017. PMID: 28551786 Review.
-
Contribution of polyol pathway to diabetes-induced oxidative stress.J Am Soc Nephrol. 2003 Aug;14(8 Suppl 3):S233-6. doi: 10.1097/01.asn.0000077408.15865.06. J Am Soc Nephrol. 2003. PMID: 12874437 Review.
Cited by
-
Aldose reductase, oxidative stress, and diabetic mellitus.Front Pharmacol. 2012 May 9;3:87. doi: 10.3389/fphar.2012.00087. eCollection 2012. Front Pharmacol. 2012. PMID: 22582044 Free PMC article.
-
Identification of pharmacokinetic markers for safflower injection using a combination of system pharmacology, multicomponent pharmacokinetics, and quantitative proteomics study.Front Pharmacol. 2022 Nov 23;13:1062026. doi: 10.3389/fphar.2022.1062026. eCollection 2022. Front Pharmacol. 2022. PMID: 36506545 Free PMC article.
-
Inactivation of Cys674 in SERCA2 increases BP by inducing endoplasmic reticulum stress and soluble epoxide hydrolase.Br J Pharmacol. 2020 Apr;177(8):1793-1805. doi: 10.1111/bph.14937. Epub 2020 Jan 30. Br J Pharmacol. 2020. PMID: 31758704 Free PMC article.
-
Mitochondrial transporter ATP binding cassette mitochondrial erythroid is a novel gene required for cardiac recovery after ischemia/reperfusion.Circulation. 2011 Aug 16;124(7):806-13. doi: 10.1161/CIRCULATIONAHA.110.003418. Epub 2011 Jul 25. Circulation. 2011. PMID: 21788586 Free PMC article.
-
Ablation of junctin or triadin is associated with increased cardiac injury following ischaemia/reperfusion.Cardiovasc Res. 2012 May 1;94(2):333-41. doi: 10.1093/cvr/cvs119. Epub 2012 Mar 12. Cardiovasc Res. 2012. PMID: 22411973 Free PMC article.
References
-
- Bolli R. Myocardial ‘stunning’ in man. Circulation. 1992 Dec;86(6):1671–91. - PubMed
-
- Kloner RA, Ellis SG, Lange R, Braunwald E. Studies of experimental coronary artery reperfusion. Effects on infarct size, myocardial function, biochemistry, ultrastructure and microvascular damage. Circulation. 1983 Aug;68(2 Pt 2):I8–15. - PubMed
-
- Dhalla NS, Panagia V, Singal PK, Makino N, Dixon IM, Eyolfson DA. Alterations in heart membrane calcium transport during the development of ischemia-reperfusion injury. J Mol Cell Cardiol. 1988 Mar;20( Suppl 2):3–13. - PubMed
-
- Guatimosim S, Dilly K, Santana LF, Saleet Jafri M, Sobie EA, Lederer WJ. Local Ca(2+) signaling and EC coupling in heart: Ca(2+) sparks and the regulation of the [Ca(2+)](i) transient. J Mol Cell Cardiol. 2002 Aug;34(8):941–50. - PubMed
-
- Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988 Jan 25;263(3):1353–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

