Bnip3 mediates permeabilization of mitochondria and release of cytochrome c via a novel mechanism
- PMID: 20025887
- PMCID: PMC2866782
- DOI: 10.1016/j.yjmcc.2009.12.004
Bnip3 mediates permeabilization of mitochondria and release of cytochrome c via a novel mechanism
Abstract
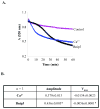
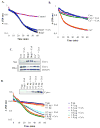
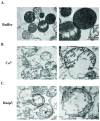
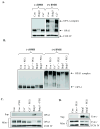
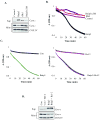
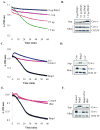
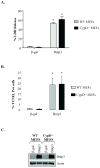
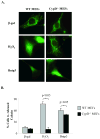
Bnip3 is a member of the BH3-only subfamily of pro-apoptotic Bcl-2 proteins and is associated with loss of cardiac myocytes after a myocardial infarction. Previous studies have demonstrated that Bnip3 induces mitochondrial dysfunction, but the mechanisms involved in this process remain unknown. In this study, we demonstrate that Bnip3 induces permeabilization of the mitochondria via a novel mechanism that is different from other BH3-only proteins. We found that Bnip3 induced mitochondrial swelling and cytochrome c release in isolated heart mitochondria in vitro. Another BH3-only protein, tBid, also caused release of cytochrome c but failed to induce swelling of mitochondria. Swelling of mitochondria is a characteristic of mitochondrial permeability transition pore (mPTP) opening, but Bnip3-mediated mitochondrial swelling was insensitive to cyclosporine A, an inhibitor of the mPTP and independent of cyclophilin D (cypD), an essential component of the mPTP. Bnip3 also induced permeabilization of the mitochondrial membranes as evident by calcein release from the matrix in both wild type (WT) and cypD deficient mouse embryonic fibroblasts (MEFs). Moreover, Bnip3 induced mitochondrial matrix remodeling and large amplitude swelling of the inner membrane, which led to disassembly of OPA1 complexes and release from the mitochondria. Thus, these studies suggest that Bnip3 mediates mitochondrial permeabilization by a novel mechanism that is different from other BH3-only proteins.
(c) 2009 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Bnip3-mediated mitochondrial autophagy is independent of the mitochondrial permeability transition pore.Autophagy. 2010 Oct;6(7):855-62. doi: 10.4161/auto.6.7.13005. Autophagy. 2010. PMID: 20668412 Free PMC article.
-
Bnip3 mediates mitochondrial dysfunction and cell death through Bax and Bak.Biochem J. 2007 Aug 1;405(3):407-15. doi: 10.1042/BJ20070319. Biochem J. 2007. PMID: 17447897 Free PMC article.
-
Impaired NF-κB signalling underlies cyclophilin D-mediated mitochondrial permeability transition pore opening in doxorubicin cardiomyopathy.Cardiovasc Res. 2020 May 1;116(6):1161-1174. doi: 10.1093/cvr/cvz240. Cardiovasc Res. 2020. PMID: 31566215 Free PMC article.
-
Bnip3 as a dual regulator of mitochondrial turnover and cell death in the myocardium.Pediatr Cardiol. 2011 Mar;32(3):267-74. doi: 10.1007/s00246-010-9876-5. Epub 2011 Jan 6. Pediatr Cardiol. 2011. PMID: 21210091 Free PMC article. Review.
-
[BNIP3 as an atypical representative of the Bcl-2 protein family. Part 1: BNIP3, a regulator of non-apoptotic programmed cell death].Postepy Hig Med Dosw (Online). 2009 Sep 10;63:409-17. Postepy Hig Med Dosw (Online). 2009. PMID: 19745227 Review. Polish.
Cited by
-
Selective autophagy as a therapeutic target for neurological diseases.Cell Mol Life Sci. 2021 Feb;78(4):1369-1392. doi: 10.1007/s00018-020-03667-9. Epub 2020 Oct 16. Cell Mol Life Sci. 2021. PMID: 33067655 Free PMC article. Review.
-
Parkin and mitophagy in cancer.Oncogene. 2017 Mar;36(10):1315-1327. doi: 10.1038/onc.2016.302. Epub 2016 Sep 5. Oncogene. 2017. PMID: 27593930 Review.
-
Multiomics Approach Reveals an Important Role of BNIP3 in Myocardial Remodeling and the Pathogenesis of Heart Failure with Reduced Ejection Fraction.Cells. 2022 May 6;11(9):1572. doi: 10.3390/cells11091572. Cells. 2022. PMID: 35563877 Free PMC article.
-
Bnip3 and AIF cooperate to induce apoptosis and cavitation during epithelial morphogenesis.J Cell Biol. 2012 Jul 9;198(1):103-14. doi: 10.1083/jcb.201111063. Epub 2012 Jul 2. J Cell Biol. 2012. PMID: 22753893 Free PMC article.
-
Impact of caloric restriction on myocardial ischaemia/reperfusion injury and new therapeutic options to mimic its effects.Br J Pharmacol. 2014 Jun;171(12):2964-92. doi: 10.1111/bph.12650. Br J Pharmacol. 2014. PMID: 24611611 Free PMC article.
References
-
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007 Jan;87(1):99–163. - PubMed
-
- Gustafsson AB, Gottlieb RA. Bcl-2 Family Members and Apoptosis, Taken to Heart. Am J Physiol Cell Physiol. 2006 Aug 30; - PubMed
-
- Graham RM, Thompson JW, Wei J, Bishopric NH, Webster KA. Regulation of bnip3 death pathways by calcium, phosphorylation, and hypoxia-reoxygenation. Antioxid Redox Signal. 2007 Sep;9(9):1309–16. - PubMed
-
- Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007 Jan;14(1):146–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

