A new steroidal 5,7-diene derivative, 3beta-hydroxyandrosta-5,7-diene-17beta-carboxylic acid, shows potent anti-proliferative activity
- PMID: 20025893
- PMCID: PMC2846116
- DOI: 10.1016/j.steroids.2009.12.004
A new steroidal 5,7-diene derivative, 3beta-hydroxyandrosta-5,7-diene-17beta-carboxylic acid, shows potent anti-proliferative activity
Erratum in
- Steroids. 2010 Dec 12;75(13-14):1164
Abstract
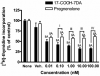
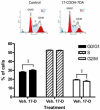
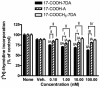
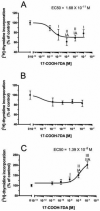
The new steroidal 5,7-diene, 3beta-hydroxyandrosta-5,7-diene-17beta-carboxylic acid (17-COOH-7DA), was synthesized from 21-acetoxypregnenolone, with the oxidative cleavage of the side chain being dependent on the presence of oxygen. In human epidermal (HaCaT) keratinocytes, 17-COOH-7DA inhibited proliferation in a dose-dependent manner, starting at a dose as low as 10(-11) M. This inhibition was accompanied by decreased expression of epidermal growth factor receptor, bcl2 and cyclin E2 mRNAs and by increased expression of involucrin mRNA. Inhibition of proliferation was associated with slowing of the cell cycle in G1/G0 phases but not with cell death. 17-COOH-7DA was significantly more potent than pregnenolone, 17-COOH-pregnenolone, 17-COOCH(3)-7DA and calcitriol. 17-COOH-7DA also inhibited proliferation of normal human epidermal melanocytes and human and hamster melanoma lines, however, with lower potency than for keratinocytes. In normal human dermal fibroblasts 17-COOH-7DA stimulated proliferation in serum-free media but inhibited it in the presence of 5% serum. 17-COOH-7DA inhibited cell colony formation of human and hamster melanoma cells, and induced monocyte-like differentiation of human HL60 leukemia cells. Thus, the new steroidal 5,7-diene, 17-COOH-7DA, can serve as an inhibitor of proliferation of normal keratinocytes and normal and malignant melanocytes, as a condition-dependent regulator of fibroblast proliferation and a stimulator of leukemia cell differentiation.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells.Am J Physiol Cell Physiol. 2011 Mar;300(3):C526-41. doi: 10.1152/ajpcell.00203.2010. Epub 2010 Dec 15. Am J Physiol Cell Physiol. 2011. PMID: 21160030 Free PMC article.
-
New steroidal oxazolines, benzoxazoles and benzimidazoles related to abiraterone and galeterone.Steroids. 2020 Jan;153:108534. doi: 10.1016/j.steroids.2019.108534. Epub 2019 Oct 31. Steroids. 2020. PMID: 31678134
-
20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes.J Cell Physiol. 2010 Apr;223(1):36-48. doi: 10.1002/jcp.21992. J Cell Physiol. 2010. PMID: 20020487 Free PMC article.
-
Discovery of novel 3-hydroxyandrosta-5,7-Diene-17-Carboxylic acid derivatives as anti-inflammatory bowel diseases (IBD) agents.Eur J Med Chem. 2021 Aug 5;220:113468. doi: 10.1016/j.ejmech.2021.113468. Epub 2021 Apr 24. Eur J Med Chem. 2021. PMID: 33933753
-
The eumelanin intermediate 5,6-dihydroxyindole-2-carboxylic acid is a messenger in the cross-talk among epidermal cells.J Invest Dermatol. 2012 Apr;132(4):1196-205. doi: 10.1038/jid.2011.457. Epub 2012 Feb 2. J Invest Dermatol. 2012. PMID: 22297637
Cited by
-
Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes.Int J Biochem Cell Biol. 2012 Nov;44(11):2003-18. doi: 10.1016/j.biocel.2012.07.027. Epub 2012 Aug 2. Int J Biochem Cell Biol. 2012. PMID: 22877869 Free PMC article.
-
Synthesis and photochemical transformation of 3β,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity.Steroids. 2011 Jan;76(1-2):193-203. doi: 10.1016/j.steroids.2010.10.009. Epub 2010 Nov 9. Steroids. 2011. PMID: 21070794 Free PMC article.
-
Cytochromes p450 and skin cancer: role of local endocrine pathways.Anticancer Agents Med Chem. 2014 Jan;14(1):77-96. doi: 10.2174/18715206113139990308. Anticancer Agents Med Chem. 2014. PMID: 23869782 Free PMC article. Review.
-
Effects of sidechain length and composition on the kinetic conversion and product distribution of vitamin D analogs determined by real-time NMR.Dermatoendocrinol. 2013 Jan 1;5(1):142-9. doi: 10.4161/derm.24339. Dermatoendocrinol. 2013. PMID: 24494047 Free PMC article.
-
New vitamin D analogs as potential therapeutics in melanoma.Expert Rev Anticancer Ther. 2012 May;12(5):585-99. doi: 10.1586/era.12.40. Expert Rev Anticancer Ther. 2012. PMID: 22594894 Free PMC article. Review.
References
-
- Tint GS, Irons M, Elias ER, Batta AK, Frieden R, Chen TS, Salen G. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N Engl J Med. 1994;330:107–13. - PubMed
-
- Shackleton CH, Roitman E, Kelley R. Neonatal urinary steroids in Smith-Lemli-Opitz syndrome associated with 7-dehydrocholesterol reductase deficiency. Steroids. 1999;64:481–90. - PubMed
-
- Shackleton C, Roitman E, Guo LW, Wilson WK, Porter FD. Identification of 7(8) and 8(9) unsaturated adrenal steroid metabolites produced by patients with 7-dehydrosterol-delta7-reductase deficiency (Smith-Lemli-Opitz syndrome) J Steroid Biochem Mol Biol. 2002;82:225–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

