NELF is a nuclear protein involved in hypothalamic GnRH neuronal migration
- PMID: 20025934
- PMCID: PMC3437992
- DOI: 10.1016/j.mce.2009.11.016
NELF is a nuclear protein involved in hypothalamic GnRH neuronal migration
Abstract
Nasal embryonic LHRH factor (NELF) has been hypothesized to participate in the migration of GnRH and olfactory neurons into the forebrain, a prerequisite for normal hypothalamic-pituitary-gonadal function in puberty and reproduction. However, the biological functions of NELF, which has no homology to any human protein, remain largely elusive. Although mRNA expression did not differ, NELF protein expression was greater in migratory than postmigratory GnRH neurons. Pituitary Nelf mRNA expression was also observed and increased 3-fold after exogenous GnRH administration. Contrary to a previous report, NELF displayed predominant nuclear localization in GnRH neurons, confirmed by mutagenesis of a putative nuclear localization signal resulting in impaired nuclear expression. NELF knockdown impaired GnRH neuronal migration of NLT cells in vitro. These findings and the identification of two putative zinc fingers suggest that NELF could be a transcription factor. Collectively, our findings implicate NELF as a nuclear protein involved in the developmental function of the reproductive axis.
Copyright 2009 Elsevier Ireland Ltd. All rights reserved.
Figures


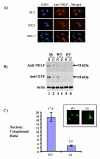
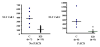

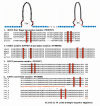
Similar articles
-
NELF knockout is associated with impaired pubertal development and subfertility.Mol Cell Endocrinol. 2015 May 15;407:26-36. doi: 10.1016/j.mce.2015.02.015. Epub 2015 Feb 27. Mol Cell Endocrinol. 2015. PMID: 25731822 Free PMC article.
-
Differential expression of nasal embryonic LHRH factor (NELF) variants in immortalized GnRH neuronal cell lines.Mol Cell Endocrinol. 2014 Mar 5;383(1-2):32-7. doi: 10.1016/j.mce.2013.11.020. Epub 2013 Dec 4. Mol Cell Endocrinol. 2014. PMID: 24316376 Free PMC article.
-
JAK/STAT signaling pathway gene expression is reduced following Nelf knockdown in GnRH neurons.Mol Cell Endocrinol. 2018 Jul 15;470:151-159. doi: 10.1016/j.mce.2017.10.009. Epub 2017 Oct 16. Mol Cell Endocrinol. 2018. PMID: 29050862
-
Factors involved in the migration of neuroendocrine hypothalamic neurons.Arch Ital Biol. 2005 Sep;143(3-4):171-8. Arch Ital Biol. 2005. PMID: 16097493 Review.
-
Molecular mechanisms of gonadotropin-releasing hormone neuronal migration.Trends Endocrinol Metab. 2004 Apr;15(3):96-102. doi: 10.1016/j.tem.2004.02.003. Trends Endocrinol Metab. 2004. PMID: 15046737 Review.
Cited by
-
Nasal embryonic LHRH factor (NELF) mutations in patients with normosmic hypogonadotropic hypogonadism and Kallmann syndrome.Fertil Steril. 2011 Apr;95(5):1613-20.e1-7. doi: 10.1016/j.fertnstert.2011.01.010. Epub 2011 Feb 15. Fertil Steril. 2011. PMID: 21300340 Free PMC article.
-
Alteration of replication protein A binding mode on single-stranded DNA by NSMF potentiates RPA phosphorylation by ATR kinase.Nucleic Acids Res. 2023 Aug 25;51(15):7936-7950. doi: 10.1093/nar/gkad543. Nucleic Acids Res. 2023. PMID: 37378431 Free PMC article.
-
Genetics of congenital hypogonadotropic hypogonadism: peculiarities and phenotype of an oligogenic disease.Hum Genet. 2021 Jan;140(1):77-111. doi: 10.1007/s00439-020-02147-1. Epub 2020 Mar 21. Hum Genet. 2021. PMID: 32200437 Review.
-
NELF knockout is associated with impaired pubertal development and subfertility.Mol Cell Endocrinol. 2015 May 15;407:26-36. doi: 10.1016/j.mce.2015.02.015. Epub 2015 Feb 27. Mol Cell Endocrinol. 2015. PMID: 25731822 Free PMC article.
-
Differential gene expression in migratory streams of cortical interneurons.Eur J Neurosci. 2011 Nov;34(10):1584-94. doi: 10.1111/j.1460-9568.2011.07896.x. Eur J Neurosci. 2011. PMID: 22103416 Free PMC article.
References
-
- Achermann JC, Meeks JJ, Jameson JL. Phenotypic spectrum of mutations in DAX-1 and SF-1. Mol Cell Endocrinol. 2001;185:17–25. - PubMed
-
- Allen MP, Xu M, Linseman DA, Pawlowski JE, Bokoch GM, Heidenreich KA, Wierman ME. Adhesion-related kinase repression of gonadotropin-releasing hormone gene expression requires Rac activation of the extracellular signal-regulated kinase pathway. J Biol Chem. 2002;277:38133–40. - PubMed
-
- Blobel G, Potter VR. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966;154:1662–5. - PubMed
-
- Cariboni A, Pimpinelli F, Colamarino S, Zaninetti R, Piccolella M, Rumio C, Piva F, Rugarli EI, Maggi R. The product of X-linked Kallmann’s syndrome gene (KAL1) affects the migratory activity of gonadotropin-releasing hormone (GnRH)-producing neurons. Hum Mol Genet. 2004;13:2781–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

