Gene delivery nanoparticles fabricated by supercritical fluid extraction of emulsions
- PMID: 20025945
- PMCID: PMC2830003
- DOI: 10.1016/j.ijpharm.2009.12.024
Gene delivery nanoparticles fabricated by supercritical fluid extraction of emulsions
Abstract
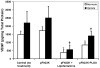
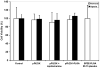
Non-viral polymeric gene delivery systems offer increased protection from nuclease degradation, enhanced plasmid DNA (pDNA) uptake, and controlled dosing to sustain the duration of pDNA action. Such gene delivery systems can be formulated from biocompatible and biodegradable polymers such as poly(D,L-lactic-co-glycolic) acid (PLGA). Experimental loading of hydrophilic macromolecules such as pDNA is low in polymeric particles. The study purpose was to develop a supercritical fluid extraction of emulsions (SFEE) process based on CO(2) for preparing pEGFP-PLGA nanoparticles with high plasmid loading and loading efficiency. Another objective was to determine the efficacy of pFlt23k, an anti-angiogenic pDNA capable of inhibiting vascular endothelial growth factor (VEGF) secretion, following nanoparticle formation using the SFEE process. Results indicated that the SFEE process allows high actual loading of pDNA (19.7%, w/w), high loading efficiency (>98%), and low residual solvents (<50 ppm), due to rapid particle formation from efficient solvent removal provided by the SFEE process. pFlt23K-PLGA nanoparticles were capable of in vitro transfection, significantly reducing secreted VEGF from human lung alveolar epithelial cells (A549) under normoxic and hypoxic conditions. pFlt23K-PLGA nanoparticles did not exhibit cytotoxicity and are of potential value in treating neovascular disorders wherein VEGF levels are elevated.
2009 Elsevier B.V. All rights reserved.
Figures


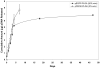
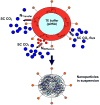
Direct mass transfer of solvent into SC CO2
Diffusion of solvent into emulsion continuous phase, subsequent mass transfer from this mixture into SC CO2.
SC CO2 movement into the emulsion droplet leading to expansion of the organic phase.
Similar articles
-
Comparative study of poly (lactic-co-glycolic acid)-poly ethyleneimine-plasmid DNA microparticles prepared using double emulsion methods.J Microencapsul. 2008 Feb;25(1):1-12. doi: 10.1080/02652040701659347. J Microencapsul. 2008. PMID: 18188727
-
Poly(L-lactide-co-glycolide) nanospheres conjugated with a nuclear localization signal for delivery of plasmid DNA.J Drug Target. 2007 Apr;15(3):190-8. doi: 10.1080/10611860601143479. J Drug Target. 2007. PMID: 17454356
-
Rational design, fabrication, characterization and in vitro testing of biodegradable microparticles that generate targeted and sustained transgene expression in HepG2 liver cells.J Drug Target. 2011 Jul;19(6):393-408. doi: 10.3109/1061186X.2010.504263. Epub 2010 Aug 3. J Drug Target. 2011. PMID: 20681752 Free PMC article.
-
[Advance in the study of poly(lactide-co-glycolide) nano/microparticles as gene vector].Yao Xue Xue Bao. 2010 Nov;45(11):1346-53. Yao Xue Xue Bao. 2010. PMID: 21361033 Review. Chinese.
-
Synthesis and characterization of PLGA nanoparticles.J Biomater Sci Polym Ed. 2006;17(3):247-89. doi: 10.1163/156856206775997322. J Biomater Sci Polym Ed. 2006. PMID: 16689015 Review.
Cited by
-
Nanomedicines for back of the eye drug delivery, gene delivery, and imaging.Prog Retin Eye Res. 2013 Sep;36:172-98. doi: 10.1016/j.preteyeres.2013.04.001. Epub 2013 Apr 17. Prog Retin Eye Res. 2013. PMID: 23603534 Free PMC article. Review.
-
Supercritical Fluid Technology: An Emphasis on Drug Delivery and Related Biomedical Applications.Adv Healthc Mater. 2017 Aug;6(16):10.1002/adhm.201700433. doi: 10.1002/adhm.201700433. Epub 2017 Jul 28. Adv Healthc Mater. 2017. PMID: 28752598 Free PMC article. Review.
-
Pharmaceutical Applications of Supercritical Fluid Extraction of Emulsions for Micro-/Nanoparticle Formation.Pharmaceutics. 2021 Nov 14;13(11):1928. doi: 10.3390/pharmaceutics13111928. Pharmaceutics. 2021. PMID: 34834343 Free PMC article. Review.
-
Enhanced endothelialization of a new stent polymer through surface enhancement and incorporation of growth factor-delivering microparticles.J Cardiovasc Transl Res. 2012 Aug;5(4):519-27. doi: 10.1007/s12265-012-9381-8. Epub 2012 May 26. J Cardiovasc Transl Res. 2012. PMID: 22639344
-
Engineering Inhalable Therapeutic Particles: Conventional and Emerging Approaches.Pharmaceutics. 2023 Nov 30;15(12):2706. doi: 10.3390/pharmaceutics15122706. Pharmaceutics. 2023. PMID: 38140047 Free PMC article. Review.
References
-
- Ayalasomayajula SP, Kompella UB. Celecoxib, a selective cyclooxygenase-2 inhibitor, inhibits retinal vascular endothelial growth factor expression and vascular leakage in a streptozotocin-induced diabetic rat model. Eur J Pharmacol. 2003;458(3):283–9. - PubMed
-
- Baldyga J, Henczka M, Shekunov B. In: Supercritical Fluid Technology for Drug Product Development. York P, Kompella UB, Shekunov BY, editors. Vol. 138. Marcel Dekker Inc.; New York: 2004. pp. 91–157.
-
- Bandi N, Kompella UB. Budesonide reduces vascular endothelial growth factor secretion and expression in airway (Calu-1) and alveolar (A549) epithelial cells. Eur J Pharmacol. 2001;425(2):109–16. - PubMed
-
- Bleich J, Muller BW. Production of drug loaded microparticles by the use of supercritical gases with the aerosol solvent extraction system (ASES) process. J Microencap. 1996;13(2):131–9. - PubMed
-
- Chattopadhyay P, Huff R, Shekunov BY. Drug encapsulation using supercritical fluid extraction of emulsions. J Pharm Sci. 2006;95(3):667–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

