Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia
- PMID: 20026066
- PMCID: PMC3806111
- DOI: 10.1053/j.gastro.2009.12.005
Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia
Abstract
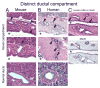
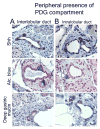
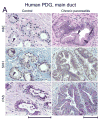
Background & aims: Pancreatic intraepithelial neoplasia (PanIN) are pancreatic cancer precursor lesions of unclear origin and significance. PanIN aberrantly express sonic hedgehog (Shh), an initiator of pancreatic cancer, and gastrointestinal mucins. A majority of PanIN are thought to arise from ducts. We identified a novel ductal compartment that is gathered in gland-like outpouches (pancreatic duct glands [PDG]) of major ducts and characterized its role in injury and metaplasia.
Methods: The ductal system was analyzed in normal pancreata and chronic pancreatitis in humans and mice. Anatomy was assessed by serial hematoxylin and eosin sections and scanning electron microscopy of corrosion casts. Expression of mucins and developmental genes and proliferation were assessed by immunohistochemistry or real-time quantitative polymerase chain reaction. Effects of Shh on ductal cells were investigated by exposure to Shh in vitro and transgenic misexpression in vivo.
Results: Three-dimensional analysis revealed blind-ending outpouches of ducts in murine and human pancreata. These PDG are morphologically and molecularly distinct from normal ducts; even in normal pancreata they display PanIN and metaplastic features, such as expression of Shh and gastric mucins. They express other developmental genes, such as Pdx-1 and Hes-1. In injury, Shh is up-regulated along with gastric mucins. Expansion of the PDG compartment results in a mucinous metaplasia. Shh promotes this transformation in vitro and in vivo.
Conclusions: PDG are distinct gland-like mucinous compartments with a distinct molecular signature. In response to injury, PDG undergo an Shh-mediated mucinous gastrointestinal metaplasia with PanIN-like features. PDG may provide a link between Shh, mucinous metaplasia, and neoplasia.
Copyright 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
None of the authors have a conflict of interest.
Figures










Comment in
-
Tracking down the hedgehog's lair in the pancreas.Gastroenterology. 2010 Mar;138(3):823-5. doi: 10.1053/j.gastro.2010.01.021. Epub 2010 Jan 25. Gastroenterology. 2010. PMID: 20100446 Free PMC article. No abstract available.
References
-
- Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. - PubMed
-
- Guerra C, Schuhmacher AJ, Canamero M, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

