Estradiol: a key biological substrate mediating the response to cocaine in female rats
- PMID: 20026119
- PMCID: PMC3621914
- DOI: 10.1016/j.yhbeh.2009.12.003
Estradiol: a key biological substrate mediating the response to cocaine in female rats
Abstract
A consistent finding in drug abuse research is that males and females show differences in their response to drugs of abuse. In women, increased plasma estradiol is associated with increased vulnerability to the psychostimulant and reinforcing effects of drugs of abuse. Our laboratory has focused on the role of estradiol in modulating the response to cocaine. We have seen that ovariectomy increases the locomotor response to a single cocaine injection, whereas estradiol exacerbates the locomotor response to repeated cocaine administration. Cocaine-induced sensitization of brain activity, as measured by fMRI, is also dependent on plasma estradiol. Moreover, we observed that although all ovariectomized rats show conditioned place preference to cocaine, it is more robust in ovariectomized rats with estradiol. Opioid receptors are enriched in brain regions associated with pleasure and reward. We find that in females, the effectiveness of kappa opioid agonists in decreasing the locomotor response to repeated cocaine varies with plasma estradiol. We also find that estradiol regulates the density of mu opioid receptors in brains areas associated with reward. These data hint that in females, estradiol modulates the behavioral effects of cocaine by regulating mu and kappa opioid signaling in mesocorticolimbic brain structures. Identifying the mechanisms that mediate differences in vulnerability to drugs of abuse may lead to effective therapeutic strategies for the treatment and prevention of addiction and relapse. We encourage health practitioners treating persons addicted to drugs to consider gender differences in response to particular pharmacotherapies, as well the sex steroid milieu of the patient.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures




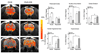



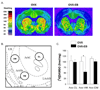
Similar articles
-
Estrogen Receptor β in the Nucleus Accumbens Regulates the Rewarding Properties of Cocaine in Female Mice.Int J Neuropsychopharmacol. 2018 Apr 1;21(4):382-392. doi: 10.1093/ijnp/pyx118. Int J Neuropsychopharmacol. 2018. PMID: 29294029 Free PMC article.
-
Estrogen receptors mediate estradiol's effect on sensitization and CPP to cocaine in female rats: role of contextual cues.Horm Behav. 2014 Feb;65(2):77-87. doi: 10.1016/j.yhbeh.2013.12.007. Epub 2013 Dec 17. Horm Behav. 2014. PMID: 24355096 Free PMC article.
-
Estradiol facilitation of cocaine-induced locomotor sensitization in female rats requires activation of mGluR5.Behav Brain Res. 2014 Sep 1;271:39-42. doi: 10.1016/j.bbr.2014.05.052. Epub 2014 Jun 2. Behav Brain Res. 2014. PMID: 24893316 Free PMC article.
-
Regulation of cocaine-related behaviours by estrogen and progesterone.Neurosci Biobehav Rev. 2022 Apr;135:104584. doi: 10.1016/j.neubiorev.2022.104584. Epub 2022 Feb 19. Neurosci Biobehav Rev. 2022. PMID: 35189163 Review.
-
Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors.Ann N Y Acad Sci. 2001 Jun;937:1-26. doi: 10.1111/j.1749-6632.2001.tb03556.x. Ann N Y Acad Sci. 2001. PMID: 11458532 Review.
Cited by
-
Sustained splenic contraction after daily cocaine administration in rats.PLoS One. 2021 Jun 4;16(6):e0252853. doi: 10.1371/journal.pone.0252853. eCollection 2021. PLoS One. 2021. PMID: 34086815 Free PMC article.
-
Testosterone is essential for cocaine sensitization in male rats.Physiol Behav. 2011 Jan 10;102(1):96-104. doi: 10.1016/j.physbeh.2010.09.025. Epub 2010 Oct 14. Physiol Behav. 2011. PMID: 20932851 Free PMC article.
-
Sex differences in opioid analgesia and addiction: interactions among opioid receptors and estrogen receptors.Mol Pain. 2013 Sep 8;9:45. doi: 10.1186/1744-8069-9-45. Mol Pain. 2013. PMID: 24010861 Free PMC article. Review.
-
Reduced sensitivity to cocaine effects and changes in mesocorticolimbic dopamine receptors in adolescent sexually active female rats.Psychopharmacology (Berl). 2025 Apr;242(4):817-834. doi: 10.1007/s00213-024-06741-3. Epub 2024 Dec 27. Psychopharmacology (Berl). 2025. PMID: 39729197
-
Lack of Sex Differences in Psychostimulant-Induced Locomotor Activity When Comparing Rats From the Same Behavioral Groups.Biol Psychiatry Glob Open Sci. 2025 Apr 25;5(5):100519. doi: 10.1016/j.bpsgos.2025.100519. eCollection 2025 Sep. Biol Psychiatry Glob Open Sci. 2025. PMID: 40612023 Free PMC article.
References
-
- Amalric M, Cline EJ, Martinez JL, Jr, Bloom FE, Koob GF. Rewarding properties of beta-endorphin as measured by conditioned place preference. Psychopharmacology (Berl) 1987;91:14–19. - PubMed
-
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. - PubMed
-
- Barrett AC, Smith ES, Picker MJ. Sex-related differences in mechanical nociception and antinociception produced by mu- and kappa-opioid receptor agonists in rats. Eur.J.Pharmacol. 2002;452:163–173. - PubMed
-
- Bartoletti M, Gaiardi M, Gubellini G, Bacchi A, Babbini M. Long-term sensitization to the excitatory effects of morphine. A motility study in post-dependent rats. Neuropharmacology. 1983;22:1193–1196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

