Towards the understanding of resistance mechanisms in clinically isolated trimethoprim-resistant, methicillin-resistant Staphylococcus aureus dihydrofolate reductase
- PMID: 20026215
- PMCID: PMC2841211
- DOI: 10.1016/j.jsb.2009.12.011
Towards the understanding of resistance mechanisms in clinically isolated trimethoprim-resistant, methicillin-resistant Staphylococcus aureus dihydrofolate reductase
Abstract
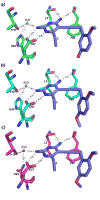
Resistance to therapeutics such as trimethoprim-sulfamethoxazole has become an increasing problem in strains of methicillin-resistant Staphylococcus aureus (MRSA). Clinically isolated trimethoprim-resistant strains reveal a double mutation, H30N/F98Y, in dihydrofolate reductase (DHFR). In order to develop novel and effective therapeutics against these resistant strains, we evaluated a series of propargyl-linked antifolate lead compounds for inhibition of the mutant enzyme. For the propargyl-linked antifolates, the F98Y mutation generates minimal (between 1.2- and 6-fold) losses of affinity and the H30N mutation generates greater losses (between 2.4- and 48-fold). Conversely, trimethoprim affinity is largely diminished by the F98Y mutation (36-fold) and is not affected by the H30N mutation. In order to elucidate a mechanism of resistance, we determined a crystal structure of a complex of this double mutant with a lead propargyl-linked antifolate. This structure suggests a resistance mechanism consistent both for the propargyl-linked class of antifolates and for trimethoprim that is based on the loss of a conserved water-mediated hydrogen bond.
Keywords: antifolates; dihydrofolate reductase; methicillin-resistant Staphylococcus aureus; trimethoprim.
(c) 2009 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Trimethoprim and other nonclassical antifolates an excellent template for searching modifications of dihydrofolate reductase enzyme inhibitors.J Antibiot (Tokyo). 2020 Jan;73(1):5-27. doi: 10.1038/s41429-019-0240-6. Epub 2019 Oct 2. J Antibiot (Tokyo). 2020. PMID: 31578455 Free PMC article. Review.
-
Crystal structures of wild-type and mutant methicillin-resistant Staphylococcus aureus dihydrofolate reductase reveal an alternate conformation of NADPH that may be linked to trimethoprim resistance.J Mol Biol. 2009 Apr 17;387(5):1298-308. doi: 10.1016/j.jmb.2009.02.045. Epub 2009 Feb 26. J Mol Biol. 2009. PMID: 19249312 Free PMC article.
-
Prospective screening of novel antibacterial inhibitors of dihydrofolate reductase for mutational resistance.Antimicrob Agents Chemother. 2012 Jul;56(7):3556-62. doi: 10.1128/AAC.06263-11. Epub 2012 Apr 9. Antimicrob Agents Chemother. 2012. PMID: 22491688 Free PMC article.
-
Chiral evasion and stereospecific antifolate resistance in Staphylococcus aureus.PLoS Comput Biol. 2022 Feb 10;18(2):e1009855. doi: 10.1371/journal.pcbi.1009855. eCollection 2022 Feb. PLoS Comput Biol. 2022. PMID: 35143481 Free PMC article.
-
Trimethoprim and brodimoprim resistance of gram-positive and gram-negative bacteria.J Chemother. 1993 Dec;5(6):458-64. J Chemother. 1993. PMID: 8195838 Review.
Cited by
-
Inhibition of antibiotic-resistant Staphylococcus aureus by the broad-spectrum dihydrofolate reductase inhibitor RAB1.Antimicrob Agents Chemother. 2010 Sep;54(9):3825-33. doi: 10.1128/AAC.00361-10. Epub 2010 Jul 6. Antimicrob Agents Chemother. 2010. PMID: 20606069 Free PMC article.
-
Trimethoprim and other nonclassical antifolates an excellent template for searching modifications of dihydrofolate reductase enzyme inhibitors.J Antibiot (Tokyo). 2020 Jan;73(1):5-27. doi: 10.1038/s41429-019-0240-6. Epub 2019 Oct 2. J Antibiot (Tokyo). 2020. PMID: 31578455 Free PMC article. Review.
-
Charged Nonclassical Antifolates with Activity Against Gram-Positive and Gram-Negative Pathogens.ACS Med Chem Lett. 2016 May 5;7(7):692-6. doi: 10.1021/acsmedchemlett.6b00120. eCollection 2016 Jul 14. ACS Med Chem Lett. 2016. PMID: 27437079 Free PMC article.
-
Propargyl-Linked Antifolates Are Potent Inhibitors of Drug-Sensitive and Drug-Resistant Mycobacterium tuberculosis.PLoS One. 2016 Aug 31;11(8):e0161740. doi: 10.1371/journal.pone.0161740. eCollection 2016. PLoS One. 2016. PMID: 27580226 Free PMC article.
-
Comparative Study of the Frech Catalyst with Two Conventional Catalysts in the Heck Synthesis of 2,4-Diaminopyrimidine-based Antibiotics.Org Prep Proced Int. 2013;45(1):66-71. doi: 10.1080/00304948.2013.743755. Org Prep Proced Int. 2013. PMID: 23788820 Free PMC article. No abstract available.
References
-
- Bhat TN, Cohen GH. OMITMAP: An electron density map suitable for the examination of errors in a macromolecular model. J Appl Cryst. 1984;17:244–248.
-
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

