Acute noxious stimulation modifies morphine effect in serotonergic but not dopaminergic midbrain areas
- PMID: 20026253
- PMCID: PMC2823975
- DOI: 10.1016/j.neuroscience.2009.12.031
Acute noxious stimulation modifies morphine effect in serotonergic but not dopaminergic midbrain areas
Abstract
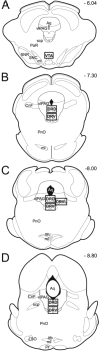
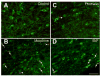
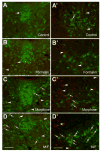
It is poorly understood if and how pain may modify the effect of opioids on neural systems that contribute to reward and addictive behavior. We hypothesized that the activation of ascending dopaminergic and serotonergic nuclei by morphine is modified by the presence of noxious stimulation. Immunohistochemical double-labeling technique with Fos was used to examine if an intraplantar formalin injection, an acute noxious input, changed the effect of morphine on dopaminergic neurons of the ventral tegmental area (VTA), and serotonergic neurons of the dorsal raphe nucleus (DR). Four groups of rats were analyzed: (1) control injected with normal saline s.c., (2) rats treated with formalin into the hind paw 30 min after normal saline injection, (3) rats injected with morphine sulfate s.c., and (4) rats treated with formalin into the hind paw 30 min after morphine injection (morphine/formalin). Following morphine injection, there was an increase in the number of dopaminergic neurons in the VTA with Fos immunolabeling. However, noxious stimulation did not detectably change morphine's effect on Fos expression in VTA dopamine neurons. In contrast, the number of serotonergic neurons containing Fos was increased in the morphine/formalin group compared to all other groups and this effect was topographically selective for the dorsal area of the DR at mid rostro-caudal levels. Therefore, morphine's activation of the VTA, which is associated with motivated behavior and reward seeking, appears similar in the context of pain. However, activation of the ascending serotonin system, which influences mood and has the capacity to modify reward pathways, appears different. In addition, these findings reveal interactions between nociceptive signaling and opioids that contrasts with the notion that opioids simply block access of nociceptive signaling to supraspinal structures.
Copyright (c) 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Visualizing acute pain-morphine interaction in descending monoamine nuclei with Fos.Brain Res. 2010 Jan 8;1306:29-38. doi: 10.1016/j.brainres.2009.10.010. Epub 2009 Oct 13. Brain Res. 2010. PMID: 19833107 Free PMC article.
-
Fos expression in serotonergic neurons in the rat brainstem following noxious stimuli: an immunohistochemical double-labelling study.J Anat. 2003 Dec;203(6):579-88. doi: 10.1046/j.1469-7580.2003.00242.x. J Anat. 2003. PMID: 14686693 Free PMC article.
-
Effects of systemic bicuculline or morphine on formalin-evoked pain-related behaviour and c-Fos expression in trigeminal nuclei after formalin injection into the lip or tongue in rats.Exp Brain Res. 2009 Jun;196(2):229-37. doi: 10.1007/s00221-009-1842-1. Epub 2009 May 22. Exp Brain Res. 2009. PMID: 19462165
-
Opiate-induced motor stimulation is regulated by gamma-aminobutyric acid type B receptors found in the ventral tegmental area in mice.Neurosci Lett. 2002 Jan 14;317(3):119-22. doi: 10.1016/s0304-3940(01)02457-0. Neurosci Lett. 2002. PMID: 11755254
-
Noxious stimulation accelerated the expression of c-fos protooncogene in cholecystokininergic and dopaminergic neurons in the ventral tegmental area.Peptides. 1993 May-Jun;14(3):561-6. doi: 10.1016/0196-9781(93)90145-7. Peptides. 1993. PMID: 8332552
Cited by
-
The role of microglia in the progression of glaucomatous neurodegeneration- a review.Int J Ophthalmol. 2018 Jan 18;11(1):143-149. doi: 10.18240/ijo.2018.01.22. eCollection 2018. Int J Ophthalmol. 2018. PMID: 29376003 Free PMC article. Review.
-
Evaluation of reward from pain relief.Ann N Y Acad Sci. 2013 Apr;1282:1-11. doi: 10.1111/nyas.12095. Epub 2013 Mar 15. Ann N Y Acad Sci. 2013. PMID: 23496247 Free PMC article. Review.
-
Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry.Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):20709-13. doi: 10.1073/pnas.1214605109. Epub 2012 Nov 26. Proc Natl Acad Sci U S A. 2012. PMID: 23184995 Free PMC article.
-
Investigation of a central nucleus of the amygdala/dorsal raphe nucleus serotonergic circuit implicated in fear-potentiated startle.Neuroscience. 2011 Apr 14;179:104-19. doi: 10.1016/j.neuroscience.2011.01.042. Epub 2011 Jan 26. Neuroscience. 2011. PMID: 21277950 Free PMC article.
References
-
- Abbott FV, Franklin KBJ, Westbrook RF. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain. 1995;60:91–102. - PubMed
-
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133(4):983–97. - PubMed
-
- Ballantyne JC. Opioid analgesia: perspectives on right use and utility. Pain Physician. 2007;10:479–491. - PubMed
-
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drungs: a meta-analysis. Neurosci Biobehavior Rev. 1995;19:39–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

