The role of NOX enzymes in ethanol-induced oxidative stress and apoptosis in mouse embryos
- PMID: 20026259
- PMCID: PMC2822117
- DOI: 10.1016/j.toxlet.2009.12.012
The role of NOX enzymes in ethanol-induced oxidative stress and apoptosis in mouse embryos
Abstract
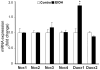
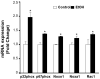
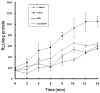
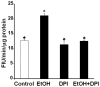
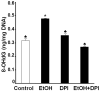
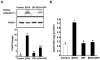
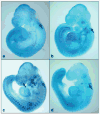
Reactive oxygen species (ROS) play an important role in ethanol-induced apoptosis and teratogenesis. However, the major sources of ROS in ethanol-exposed embryos have remained undefined. This study was conducted to determine the role of NADPH oxidase (NOX) in ethanol-induced oxidative stress and apoptosis in mouse embryos. Analyses of mRNA expression indicated that ethanol treatment resulted in a significant increase in mRNA expression of NOX catalytic subunit Duox-1 in gestational day 9 (GD 9:0) mouse embryos. Ethanol exposure also resulted in significant increases in mRNA expression of NOX regulatory subunits, p22phox, p67phox, NOXA1 and NOXO1. In addition, a significant increase in NOX enzyme activity was found in the ethanol-exposed embryos as compared to controls. Co-treatment with the NOX inhibitor, diphenyleneiodonium (DPI), significantly prevented ethanol-induced increases in NOX enzyme activity, ROS generation and oxidative DNA damage in ethanol-exposed embryos. DPI treatment also resulted in a reduction in caspase-3 activation, decreased caspase-3 activity and diminished prevalence of apoptosis in ethanol-exposed embryos. These results support the hypothesis that NOX is a critical source of ROS in ethanol-exposed embryos and that it plays an important role in ethanol-induced oxidative stress and pathogenesis.
Copyright 2009 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration.J Neuroinflammation. 2012 Jan 12;9:5. doi: 10.1186/1742-2094-9-5. J Neuroinflammation. 2012. PMID: 22240163 Free PMC article.
-
Enhanced NADPH oxidases and reactive oxygen species in the mechanism of methanol-initiated protein oxidation and embryopathies in vivo and in embryo culture.Arch Toxicol. 2016 Mar;90(3):717-30. doi: 10.1007/s00204-015-1482-0. Epub 2015 Mar 1. Arch Toxicol. 2016. PMID: 25726414
-
Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: implications for the prevention of fetal alcohol spectrum disorders.Antioxid Redox Signal. 2008 Dec;10(12):2023-33. doi: 10.1089/ars.2007.2019. Antioxid Redox Signal. 2008. PMID: 18759561 Free PMC article.
-
Molecular insights of NADPH oxidases and its pathological consequences.Cell Biochem Funct. 2021 Mar;39(2):218-234. doi: 10.1002/cbf.3589. Epub 2020 Sep 25. Cell Biochem Funct. 2021. PMID: 32975319 Review.
-
Organizers and activators: Cytosolic Nox proteins impacting on vascular function.Free Radic Biol Med. 2017 Aug;109:22-32. doi: 10.1016/j.freeradbiomed.2017.03.017. Epub 2017 Mar 21. Free Radic Biol Med. 2017. PMID: 28336130 Review.
Cited by
-
Bacterial nanocellulose/calcium alginate hydrogel for the treatment of burns.Acta Cir Bras. 2024 Jul 15;39:e393324. doi: 10.1590/acb393324. eCollection 2024. Acta Cir Bras. 2024. PMID: 39016358 Free PMC article.
-
Oxidative Stress as a Common Key Event in Developmental Neurotoxicity.Oxid Med Cell Longev. 2021 Jul 19;2021:6685204. doi: 10.1155/2021/6685204. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34336113 Free PMC article. Review.
-
N-Acetylcysteine prevents the decreases in cardiac collagen I/III ratio and systolic function in neonatal mice with prenatal alcohol exposure.Toxicol Lett. 2019 Oct 15;315:87-95. doi: 10.1016/j.toxlet.2019.08.010. Epub 2019 Aug 16. Toxicol Lett. 2019. PMID: 31425726 Free PMC article.
-
Nicotinamide adenine dinucleotide phosphate oxidase is differentially regulated in normal myometrium versus leiomyoma.Reprod Sci. 2014 Sep;21(9):1145-52. doi: 10.1177/1933719114522552. Epub 2014 Feb 11. Reprod Sci. 2014. PMID: 24520084 Free PMC article.
-
Cdc42-dependent activation of NADPH oxidase is involved in ethanol-induced neuronal oxidative stress.PLoS One. 2012;7(5):e38075. doi: 10.1371/journal.pone.0038075. Epub 2012 May 25. PLoS One. 2012. PMID: 22662267 Free PMC article.
References
-
- Abdelrahman M, Mazzon E, Bauer M, Bauer I, Delbosc S, Cristol JP, Patel NS, Cuzzocrea S, Thiemermann C. Inhibitors of NADPH oxidase reduce the organ injury in hemorrhagic shock. Shock. 2005;23:107–114. - PubMed
-
- Abel EL, Sokol RJ. Fetal alcohol syndrome is now leading cause of mental retardation. Lancet. 1986;2:1222. - PubMed
-
- Adachi J, Mizoi Y, Fukunaga T, Ogawa Y, Ueno Y, Imamichi H. Degrees of Alcohol-Intoxication in 117 Hospitalized Cases. Journal of Studies on Alcohol. 1991;52:448–453. - PubMed
-
- Babior BM. The leukocyte NADPH oxidase. Isr Med Assoc J. 2002;4:1023–1024. - PubMed
-
- Bayraktutan U, Draper N, Lang D, Shah AM. Expression of a functional neutrophil-type NADPH oxidase in cultured rat coronary microvascular endothelial cells. Cardiovascular Research. 1998;38:256–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

