Life and death of a BiP substrate
- PMID: 20026282
- PMCID: PMC2883687
- DOI: 10.1016/j.semcdb.2009.12.008
Life and death of a BiP substrate
Abstract
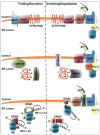
BiP is the mammalian endoplasmic reticulum (ER) Hsp70 orthologue that plays a major role in all functions of this organelle including the seemingly opposing functions of aiding the maturation of unfolded nascent proteins and identifying and targeting chronically unfolded proteins for degradation. The recent identification of mammalian BiP co-factors combined with delineation of the ER degradation machinery and data suggesting that the ER is subdivided into unique regions helps explain how these different functions can occur in the same organelle and raises some unresolved issues.
Figures

References
-
- Hammond C, Helenius A. Quality control in the secretory pathway. Curr Opin Cell Biol. 1995;7:523–9. - PubMed
-
- Jarosch E, Lenk U, Sommer T. Endoplasmic reticulum-associated protein degradation. Int Rev Cytol. 2003;223:39–81. - PubMed
-
- Hendershot LM. BiP is a master regulator of ER function. Mt Sinai J Med. 2004;71:289–97. - PubMed
-
- Molinari M, Helenius A. Chaperone selection during glycoprotein translocation into the endoplasmic reticulum. Science. 2000;288:331–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

