Fatty acid 2-Hydroxylation in mammalian sphingolipid biology
- PMID: 20026285
- PMCID: PMC2826524
- DOI: 10.1016/j.bbalip.2009.12.004
Fatty acid 2-Hydroxylation in mammalian sphingolipid biology
Abstract
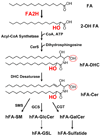
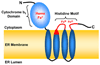
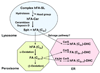
2-Hydroxy fatty acids (hFA) are important components of a subset of mammalian sphingolipids. The presence of hFA in sphingolipids is best described in the nervous system, epidermis, and kidney. However, the literature also indicates that various hFA-sphingolipids are present in additional tissues and cell types, as well as in tumors. Biosynthesis of hFA-sphingolipids requires fatty acid 2-hydroyxlase, and degradation of hFA-sphingolipids depends, at least in part, on lysosomal acid ceramidase and the peroxisomal fatty acid alpha-oxidation pathway. Mutations in the fatty acid 2-hydroxylase gene, FA2H, have been associated with leukodystrophy and spastic paraparesis in humans, underscoring the importance of hFA-sphingolipids in the nervous system. In the epidermis, hFA-ceramides are essential for the permeability barrier function. Physiological function of hFA-sphingolipids in other organs remains largely unknown. Recent evidence indicates that hFA-sphingolipids have specific roles in cell signaling.
Keywords: FA2H; Fatty acid 2-hydroxylase; fatty acid alpha-hydroxylase; hydroxy fatty acid; hydroxy sphingolipids.
2009 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
2'-Hydroxy ceramide in membrane homeostasis and cell signaling.Adv Biol Regul. 2014 Jan;54:223-30. doi: 10.1016/j.jbior.2013.09.012. Epub 2013 Oct 8. Adv Biol Regul. 2014. PMID: 24139861 Free PMC article. Review.
-
Fatty acid 2-hydroxylase regulates cAMP-induced cell cycle exit in D6P2T schwannoma cells.J Lipid Res. 2009 Jun;50(6):1203-8. doi: 10.1194/jlr.M800666-JLR200. Epub 2009 Jan 22. J Lipid Res. 2009. PMID: 19171550 Free PMC article.
-
Fatty Acid 2-Hydroxylase and 2-Hydroxylated Sphingolipids: Metabolism and Function in Health and Diseases.Int J Mol Sci. 2023 Mar 3;24(5):4908. doi: 10.3390/ijms24054908. Int J Mol Sci. 2023. PMID: 36902339 Free PMC article. Review.
-
Fatty acid 2-hydroxylase, encoded by FA2H, accounts for differentiation-associated increase in 2-OH ceramides during keratinocyte differentiation.J Biol Chem. 2007 May 4;282(18):13211-9. doi: 10.1074/jbc.M611562200. Epub 2007 Mar 12. J Biol Chem. 2007. PMID: 17355976 Clinical Trial.
-
FA2H-dependent fatty acid 2-hydroxylation in postnatal mouse brain.J Lipid Res. 2006 Dec;47(12):2772-80. doi: 10.1194/jlr.M600362-JLR200. Epub 2006 Sep 23. J Lipid Res. 2006. PMID: 16998236
Cited by
-
Arabidopsis sphingolipid fatty acid 2-hydroxylases (AtFAH1 and AtFAH2) are functionally differentiated in fatty acid 2-hydroxylation and stress responses.Plant Physiol. 2012 Jul;159(3):1138-48. doi: 10.1104/pp.112.199547. Epub 2012 May 25. Plant Physiol. 2012. PMID: 22635113 Free PMC article.
-
Novel function of FAXDC2 in megakaryopoiesis.Blood Cancer J. 2016 Sep 30;6(9):e478. doi: 10.1038/bcj.2016.87. Blood Cancer J. 2016. PMID: 27689744 Free PMC article.
-
Plasma Metabolomics and Lipidomics Differentiate Obese Individuals by Peripheral Neuropathy Status.J Clin Endocrinol Metab. 2022 Mar 24;107(4):1091-1109. doi: 10.1210/clinem/dgab844. J Clin Endocrinol Metab. 2022. PMID: 34878536 Free PMC article.
-
2'-Hydroxy ceramide in membrane homeostasis and cell signaling.Adv Biol Regul. 2014 Jan;54:223-30. doi: 10.1016/j.jbior.2013.09.012. Epub 2013 Oct 8. Adv Biol Regul. 2014. PMID: 24139861 Free PMC article. Review.
-
The Link between Gaucher Disease and Parkinson's Disease Sheds Light on Old and Novel Disorders of Sphingolipid Metabolism.Int J Mol Sci. 2019 Jul 5;20(13):3304. doi: 10.3390/ijms20133304. Int J Mol Sci. 2019. PMID: 31284408 Free PMC article. Review.
References
-
- Deuel HJ. Chemistry of the Phosphatides and Cerebrosides. In: Deuel HJ, editor. The Lipids, Their Chemistry and Biochemistry. vol. 1. New York: Chemistry, Interscience Publishers; 1951. pp. 405–496.
-
- Kishimoto Y, Radin NS. Occurrence of 2-Hydroxy Fatty Acids in Animal Tissues. J Lipid Res. 1963;4:139–143. - PubMed
-
- Hoshi M, Kishimoto Y. Synthesis of cerebronic acid from lignoceric acid by rat brain preparation. Some properties and distribution of the -hydroxylation system. J Biol Chem. 1973;248:4123–4130. - PubMed
-
- Akanuma H, Kishimoto Y. Synthesis of ceramides and cerebrosides containing both alpha-hydroxy and nonhydroxy fatty acids from lignoceroyl-CoA by rat brain microsomes. J Biol Chem. 1979;254:1050–1060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

