Interaction of the regulatory subunit of the cAMP-dependent protein kinase with PATZ1 (ZNF278)
- PMID: 20026299
- PMCID: PMC2812659
- DOI: 10.1016/j.bbrc.2009.12.026
Interaction of the regulatory subunit of the cAMP-dependent protein kinase with PATZ1 (ZNF278)
Abstract
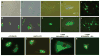
The effects of cAMP in cell are predominantly mediated by the cAMP-dependent protein kinase (PKA), which is composed of two genetically distinct subunits, catalytic (C) and regulatory (R), forming a tetrameric holoenzyme R(2)C(2). The only known function for the R subunit is that of inhibiting the activity of the C subunit kinase. It has been shown that overexpression of RIalpha, but not the C subunit kinase, is associated with neoplastic transformation. In addition, it has also been demonstrated that mutation in the RIalpha, but not the C subunit is associated with increased resistance to the DNA-damaging anticancer drug cisplatin, thus suggesting that the RIalpha subunit of PKA may have functions independent of the kinase. We show here that the RIalpha subunit interacts with a BTB/POZ domain zinc-finger transcription factor, PATZ1 (ZNF278), and co-expression with RIalpha results in its sequestration in the cytoplasm. The cytoplasmic/nuclear translocation is inducible by cAMP. C-terminus deletion abolishes PATZ1 interaction with RIalpha and results in its localization in the nucleus. PATZ1 transactivates the cMyc promoter and the presence of cAMP and co-expression with RIalpha modulates its transactivation. Moreover, PATZ1 is aberrantly expressed in cancer. Taken together, our results showed a potentially novel mechanism of cAMP signaling mediated through the interaction of RIalpha with PATZ1 that is independent of the kinase activity of PKA, and the aberrant expression of PATZ1 in cancer point to its role in cell growth regulation.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures


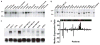
Similar articles
-
Structure of a PKA RIα Recurrent Acrodysostosis Mutant Explains Defective cAMP-Dependent Activation.J Mol Biol. 2016 Dec 4;428(24 Pt B):4890-4904. doi: 10.1016/j.jmb.2016.10.033. Epub 2016 Nov 5. J Mol Biol. 2016. PMID: 27825928 Free PMC article.
-
Cardiac ischemia-reperfusion injury induces ROS-dependent loss of PKA regulatory subunit RIα.Am J Physiol Heart Circ Physiol. 2019 Dec 1;317(6):H1231-H1242. doi: 10.1152/ajpheart.00237.2019. Epub 2019 Nov 1. Am J Physiol Heart Circ Physiol. 2019. PMID: 31674811 Free PMC article.
-
Phosphorylation of protein kinase A (PKA) regulatory subunit RIα by protein kinase G (PKG) primes PKA for catalytic activity in cells.J Biol Chem. 2018 Mar 23;293(12):4411-4421. doi: 10.1074/jbc.M117.809988. Epub 2018 Jan 29. J Biol Chem. 2018. PMID: 29378851 Free PMC article.
-
The POZ/BTB and AT-Hook Containing Zinc Finger 1 (PATZ1) Transcription Regulator: Physiological Functions and Disease Involvement.Int J Mol Sci. 2017 Nov 24;18(12):2524. doi: 10.3390/ijms18122524. Int J Mol Sci. 2017. PMID: 29186807 Free PMC article. Review.
-
Chemoprevention with protein kinase A RIalpha antisense in DMBA-mammary carcinogenesis.Ann N Y Acad Sci. 2005 Nov;1058:255-64. doi: 10.1196/annals.1359.038. Ann N Y Acad Sci. 2005. PMID: 16394142 Review.
Cited by
-
PATZ1 acts as a tumor suppressor in thyroid cancer via targeting p53-dependent genes involved in EMT and cell migration.Oncotarget. 2015 Mar 10;6(7):5310-23. doi: 10.18632/oncotarget.2776. Oncotarget. 2015. PMID: 25595894 Free PMC article.
-
The dosage of Patz1 modulates reprogramming process.Sci Rep. 2014 Dec 17;4:7519. doi: 10.1038/srep07519. Sci Rep. 2014. PMID: 25515777 Free PMC article.
-
PATZ1 knockdown enhances malignant phenotype in thyroid epithelial follicular cells and thyroid cancer cells.Oncotarget. 2017 Aug 2;8(47):82754-82772. doi: 10.18632/oncotarget.19787. eCollection 2017 Oct 10. Oncotarget. 2017. PMID: 29137300 Free PMC article.
-
PATZ1 Is a DNA Damage-Responsive Transcription Factor That Inhibits p53 Function.Mol Cell Biol. 2015 May;35(10):1741-53. doi: 10.1128/MCB.01475-14. Epub 2015 Mar 9. Mol Cell Biol. 2015. PMID: 25755280 Free PMC article.
-
BTB protein family and human breast cancer: signaling pathways and clinical progress.J Cancer Res Clin Oncol. 2023 Nov;149(17):16213-16229. doi: 10.1007/s00432-023-05314-9. Epub 2023 Sep 8. J Cancer Res Clin Oncol. 2023. PMID: 37682360 Free PMC article. Review.
References
-
- Chin KV, Yang WL, Ravatn R, Kita T, Reitman E, Vettori D, et al. Reinventing the wheel of cyclic AMP: novel mechanisms of cAMP signaling. Ann N Y Acad Sci. 2002;968:49–64. - PubMed
-
- Kirschner LS, Yin Z, Jones GN, Mahoney E. Mouse models of altered protein kinase A signaling. Endocr Relat Cancer. 2009;16:773–93. - PubMed
-
- Cho-Chung YS. cAMP signaling in cancer genesis and treatment. Cancer Treat Res. 2003;115:123–43. - PubMed
-
- Francis SH, Corbin JD. Cyclic nucleotide-dependent protein kinases: intracellular receptors for cAMP and cGMP action. Crit Rev Clin Lab Sci. 1999;36:275–328. - PubMed
-
- Edelman AM, Blumenthal DK, Krebs EG. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

