Isolation and characterization of chicken lung mesenchymal stromal cells and their susceptibility to avian influenza virus
- PMID: 20026347
- PMCID: PMC7124449
- DOI: 10.1016/j.dci.2009.12.008
Isolation and characterization of chicken lung mesenchymal stromal cells and their susceptibility to avian influenza virus
Abstract
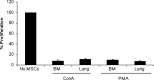
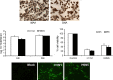
In this study, we isolated and characterized mesenchymal stromal cells (MSCs) from the lungs of 1- to 2-week-old chickens. Microscopically, the cultured cells showed fibroblast-like morphology. Phenotypically these cells expressed CD44, CD90, CD105 and the transcription factor PouV, which has been shown to be critical for stem cell self-renewal and pluripotency. The multipotency of chicken MSCs was demonstrated by their ability to undergo adipogenic and osteogenic differentiation. Like chicken bone marrow MSCs and mammalian MSCs, chicken lung MSCs had immunoregulatory activity and profoundly suppressed the proliferative capacity of T cells in response to a mitogenic stimulus. Next, we examined the susceptibility of these cells to H1N1 and H9N5 avian influenza (AI) viruses. The lung MSCs were shown to express known influenza virus alpha-2,3 and alpha-2,6 sialic acid receptors and to support replication of both the avian H1N1 and avian H9N5 influenza strains. Viral infection of MSCs resulted in cell lysis and cytokine and chemokine production. Further characterization of lung MSCs in chicken and other mammalian species may help in understanding the pathogenesis of infectious and non-infectious lung diseases and the mechanisms of lung injury repair.
Figures


References
-
- Friedenstein A.J., Deriglasova U.F., Kulagina N.N., Panasuk A.F., Rudakowa S.F., Luria E.A. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2(2):83–92. - PubMed
-
- Iyer S.S., Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther. 2008;8(5):569–581. - PubMed
-
- Awad H.A., Boivin G.P., Dressler M.R., Smith F.N., Young R.G., Butler D.L. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21(3):420–431. - PubMed
-
- Noel D., Djouad F., Jorgense C. Regenerative medicine through mesenchymal stem cells for bone and cartilage repair. Curr Opin Invest Drugs. 2002;3(7):1000–1004. - PubMed
-
- Pelttari K., Steck E., Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39(Suppl. 1):S58–S65. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

