Phospholipid and sphingolipid metabolism in Leishmania
- PMID: 20026359
- PMCID: PMC2815228
- DOI: 10.1016/j.molbiopara.2009.12.004
Phospholipid and sphingolipid metabolism in Leishmania
Abstract
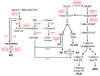
In many eukaryotes, phospholipids (PLs) and sphingolipids (SLs) are abundant membrane components and reservoirs for important signaling molecules. In Leishmania, the composition, metabolism, and function of PLs and SLs differ significantly from those in mammalian cells. Although only a handful of enzymes have been experimentally characterized, available data suggest many steps of PL/SL metabolism are critical for Leishmania viability and/or virulence, and could be a source for new drug targets. Further studies of genes involved in the synthesis (de novo and salvage) and degradation of PLs and SLs will reveal their diverse effects on Leishmania pathogenesis.
Figures

Similar articles
-
Redirection of sphingolipid metabolism toward de novo synthesis of ethanolamine in Leishmania.EMBO J. 2007 Feb 21;26(4):1094-104. doi: 10.1038/sj.emboj.7601565. Epub 2007 Feb 8. EMBO J. 2007. PMID: 17290222 Free PMC article.
-
Sphingolipids in parasitic protozoa.Adv Exp Med Biol. 2010;688:238-48. doi: 10.1007/978-1-4419-6741-1_17. Adv Exp Med Biol. 2010. PMID: 20919659 Free PMC article. Review.
-
Complex Interplay between Sphingolipid and Sterol Metabolism Revealed by Perturbations to the Leishmania Metabolome Caused by Miltefosine.Antimicrob Agents Chemother. 2018 Apr 26;62(5):e02095-17. doi: 10.1128/AAC.02095-17. Print 2018 May. Antimicrob Agents Chemother. 2018. PMID: 29463533 Free PMC article.
-
Leishmania salvage and remodelling of host sphingolipids in amastigote survival and acidocalcisome biogenesis.Mol Microbiol. 2005 Mar;55(5):1566-78. doi: 10.1111/j.1365-2958.2005.04493.x. Mol Microbiol. 2005. PMID: 15720561 Free PMC article.
-
Phospholipids and Sphingolipids in Osteoarthritis.Biomolecules. 2025 Feb 8;15(2):250. doi: 10.3390/biom15020250. Biomolecules. 2025. PMID: 40001553 Free PMC article. Review.
Cited by
-
Phosphatidylcholine synthesis through cholinephosphate cytidylyltransferase is dispensable in Leishmania major.Sci Rep. 2019 May 20;9(1):7602. doi: 10.1038/s41598-019-44086-6. Sci Rep. 2019. PMID: 31110206 Free PMC article.
-
Expanded genome-wide comparisons give novel insights into population structure and genetic heterogeneity of Leishmania tropica complex.PLoS Negl Trop Dis. 2020 Sep 18;14(9):e0008684. doi: 10.1371/journal.pntd.0008684. eCollection 2020 Sep. PLoS Negl Trop Dis. 2020. PMID: 32946436 Free PMC article.
-
In vitro infectivity and differential gene expression of Leishmania infantum metacyclic promastigotes: negative selection with peanut agglutinin in culture versus isolation from the stomodeal valve of Phlebotomus perniciosus.BMC Genomics. 2016 May 20;17:375. doi: 10.1186/s12864-016-2672-8. BMC Genomics. 2016. PMID: 27206922 Free PMC article.
-
BluePort: a platform to study the eosinophilic response of mice to the bite of a vector of Leishmania parasites, Lutzomyia longipalpis sand flies.PLoS One. 2010 Oct 27;5(10):e13546. doi: 10.1371/journal.pone.0013546. PLoS One. 2010. PMID: 21048957 Free PMC article.
-
The Glycerol-3-Phosphate Acyltransferase TbGAT is Dispensable for Viability and the Synthesis of Glycerolipids in Trypanosoma brucei.J Eukaryot Microbiol. 2016 Sep;63(5):598-609. doi: 10.1111/jeu.12309. Epub 2016 Mar 8. J Eukaryot Microbiol. 2016. PMID: 26909872 Free PMC article.
References
-
- Majerus PW, et al. The metabolism of phosphoinositide-derived messenger molecules. Science. 1986;234:1519–1526. - PubMed
-
- Chakraborti S. Phospholipase A(2) isoforms: a perspective. Cell Signal. 2003;15:637–665. - PubMed
-
- Divecha N, Irvine RF. Phospholipid signaling. Cell. 1995;80:269–278. - PubMed
-
- Coppolino MG, et al. Inhibition of phosphatidylinositol-4-phosphate 5-kinase Ialpha impairs localized actin remodeling and suppresses phagocytosis. J Biol Chem. 2002;277:43849–43857. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

