Characterizing molecular diffusion in the lens capsule
- PMID: 20026402
- PMCID: PMC2849862
- DOI: 10.1016/j.matbio.2009.12.004
Characterizing molecular diffusion in the lens capsule
Abstract
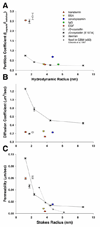
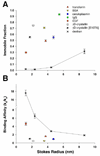
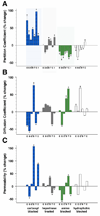
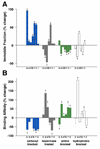
The lens capsule compartmentalizes the cells of the avascular lens from other ocular tissues. Small molecules required for lens cell metabolism, such as glucose, salts, and waste products, freely pass through the capsule. However, the lens capsule is selectively permeable to proteins such as growth hormones and substrate carriers which are required for proper lens growth and development. We used fluorescence recovery after photobleaching (FRAP) to characterize the diffusional behavior of various sized dextrans (3, 10, 40, 150, and 250 kDa) and proteins endogenous to the lens environment (EGF, gammaD-crystallin, BSA, transferrin, ceruloplasmin, and IgG) within the capsules of whole living lenses. We found that proteins had dramatically different diffusion and partition coefficients as well as capsule matrix binding affinities than similar sized dextrans, but they had comparable permeabilities. We also found ionic interactions between proteins and the capsule matrix significantly influence permeability and binding affinity, while hydrophobic interactions had less of an effect. The removal of a single anionic residue from the surface of a protein, gammaD-crystallin [E107A], significantly altered its permeability and matrix binding affinity in the capsule. Our data indicated that permeabilities and binding affinities in the lens capsule varied between individual proteins and cannot be predicted by isoelectric points or molecular size alone.
Keywords: FRAP; basement membrane; binding affinity; diffusion coefficient; lens capsule; partition coefficient; permeability.
Copyright 2009 International Society of Matrix Biology. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Age-dependency of molecular diffusion in the human anterior lens capsule assessed using fluorescence recovery after photobleaching.Mol Vis. 2019 Oct 14;25:593-xxx. eCollection 2019. Mol Vis. 2019. PMID: 31741652 Free PMC article.
-
The lens capsule.Exp Eye Res. 2009 Feb;88(2):151-64. doi: 10.1016/j.exer.2008.08.002. Epub 2008 Aug 16. Exp Eye Res. 2009. PMID: 18773892 Free PMC article. Review.
-
Determination of human lens capsule permeability and its feasibility as a replacement for Bruch's membrane.Biomaterials. 2006 Mar;27(8):1670-8. doi: 10.1016/j.biomaterials.2005.09.008. Epub 2005 Sep 30. Biomaterials. 2006. PMID: 16199085
-
Alterations in the lens capsule contribute to cataractogenesis in SPARC-null mice.J Cell Sci. 2002 Jul 1;115(Pt 13):2747-56. doi: 10.1242/jcs.115.13.2747. J Cell Sci. 2002. PMID: 12077365
-
Lens capsule as a model to study type IV collagen.Connect Tissue Res. 2014 Jan-Feb;55(1):8-12. doi: 10.3109/03008207.2013.867337. Connect Tissue Res. 2014. PMID: 24437599 Free PMC article. Review.
Cited by
-
Development of diagnostic and treatment strategies for glaucoma through understanding and modification of scleral and lamina cribrosa connective tissue.Cell Tissue Res. 2013 Aug;353(2):231-44. doi: 10.1007/s00441-013-1603-0. Epub 2013 Mar 28. Cell Tissue Res. 2013. PMID: 23535950 Free PMC article. Review.
-
Postponement of the opacification of lentoid bodies derived from human induced pluripotent stem cells after lanosterol treatment-the first use of the lens aging model in vitro in cataract drug screening.Front Pharmacol. 2022 Aug 19;13:959978. doi: 10.3389/fphar.2022.959978. eCollection 2022. Front Pharmacol. 2022. PMID: 36059984 Free PMC article.
-
Lens capsule structure assessed with atomic force microscopy.Mol Vis. 2015 Mar 15;21:316-23. eCollection 2015. Mol Vis. 2015. PMID: 25814829 Free PMC article.
-
A gradient of matrix-bound FGF-2 and perlecan is available to lens epithelial cells.Exp Eye Res. 2014 Mar;120(100):10-4. doi: 10.1016/j.exer.2013.12.004. Epub 2013 Dec 14. Exp Eye Res. 2014. PMID: 24341990 Free PMC article.
-
Whole mount staining of lenses for visualization of lens epithelial cell proteins.MethodsX. 2021 May 6;8:101376. doi: 10.1016/j.mex.2021.101376. eCollection 2021. MethodsX. 2021. PMID: 34430272 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

