Metabolic turnover analysis by a combination of in vivo 13C-labelling from 13CO2 and metabolic profiling with CE-MS/MS reveals rate-limiting steps of the C3 photosynthetic pathway in Nicotiana tabacum leaves
- PMID: 20026474
- PMCID: PMC2826653
- DOI: 10.1093/jxb/erp374
Metabolic turnover analysis by a combination of in vivo 13C-labelling from 13CO2 and metabolic profiling with CE-MS/MS reveals rate-limiting steps of the C3 photosynthetic pathway in Nicotiana tabacum leaves
Abstract
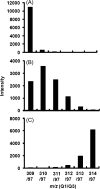
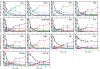
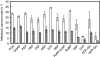
Understanding of the control of metabolic pathways in plants requires direct measurement of the metabolic turnover rate. Sugar phosphate metabolism, including the Calvin cycle, is the primary pathway in C(3) photosynthesis, the dynamic status of which has not been assessed quantitatively in the leaves of higher plants. Since the flux of photosynthetic carbon metabolism is affected by the CO(2) fixation rate in leaves, a novel in vivo (13)C-labelling system was developed with (13)CO(2) for the kinetic determination of metabolic turnover that was the time-course of the (13)C-labelling ratio in each metabolite. The system is equipped with a gas-exchange chamber that enables real-time monitoring of the CO(2) fixation rate and a freeze-clamp that excises a labelled leaf concurrently with quenching the metabolic reactions by liquid nitrogen within the photosynthesis chamber. Kinetic measurements were performed by detecting mass isotopomer abundance with capillary electrophoresis-tandem mass spectrometry. The multiple reaction monitoring method was optimized for the determination of each compound for sensitive detection because the amount of some sugar phosphates in plant cells is extremely small. Our analytical system enabled the in vivo turnover of sugar phosphates to be monitored in fresh tobacco (Nicotiana tabacum) leaves, which revealed that the turnover rate of glucose-1-phosphate (G1P) was significantly lower than that of other sugar phosphates, including glucose-6-phosphate (G6P). The pool size of G1P is 12 times lower than that of G6P. These results indicate that the conversion of G6P to G1P is one of the rate-limiting steps in the sugar phosphate pathway.
Figures

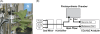

Similar articles
-
Stable Isotope Labeling and Quantification of Photosynthetic Metabolites.Methods Mol Biol. 2024;2790:439-466. doi: 10.1007/978-1-0716-3790-6_24. Methods Mol Biol. 2024. PMID: 38649586
-
Metabolic Profiling with Gas Chromatography-Mass Spectrometry and Capillary Electrophoresis-Mass Spectrometry Reveals the Carbon-Nitrogen Status of Tobacco Leaves Across Different Planting Areas.J Proteome Res. 2016 Feb 5;15(2):468-76. doi: 10.1021/acs.jproteome.5b00807. Epub 2016 Jan 27. J Proteome Res. 2016. PMID: 26784525
-
13CO2 as a universal metabolic tracer in isotopologue perturbation experiments.Phytochemistry. 2007 Aug-Sep;68(16-18):2273-89. doi: 10.1016/j.phytochem.2007.03.034. Epub 2007 May 15. Phytochemistry. 2007. PMID: 17507062
-
(12)C/(13)C fractionations in plant primary metabolism.Trends Plant Sci. 2011 Sep;16(9):499-506. doi: 10.1016/j.tplants.2011.05.010. Epub 2011 Jun 24. Trends Plant Sci. 2011. PMID: 21705262 Review.
-
Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field.J Exp Bot. 2009;60(8):2249-70. doi: 10.1093/jxb/erp036. Epub 2009 Apr 23. J Exp Bot. 2009. PMID: 19395391 Review.
Cited by
-
Dynamic metabolic profiling of the marine microalga Chlamydomonas sp. JSC4 and enhancing its oil production by optimizing light intensity.Biotechnol Biofuels. 2015 Mar 18;8:48. doi: 10.1186/s13068-015-0226-y. eCollection 2015. Biotechnol Biofuels. 2015. PMID: 25802553 Free PMC article.
-
Sphingolipid Long-Chain Base Phosphate Degradation Can Be a Rate-Limiting Step in Long-Chain Base Homeostasis.Front Plant Sci. 2022 Jun 15;13:911073. doi: 10.3389/fpls.2022.911073. eCollection 2022. Front Plant Sci. 2022. PMID: 35783987 Free PMC article.
-
Non-canonical plant metabolism.Nat Plants. 2025 Apr;11(4):696-708. doi: 10.1038/s41477-025-01965-3. Epub 2025 Mar 31. Nat Plants. 2025. PMID: 40164785 Review.
-
New Insight into the Role of the Calvin Cycle: Reutilization of CO2 Emitted through Sugar Degradation.Sci Rep. 2015 Jul 1;5:11617. doi: 10.1038/srep11617. Sci Rep. 2015. PMID: 26130086 Free PMC article.
-
Combination of long-term 13CO2 labeling and isotopolog profiling allows turnover analysis of photosynthetic pigments in Arabidopsis leaves.Plant Methods. 2022 Oct 1;18(1):114. doi: 10.1186/s13007-022-00946-3. Plant Methods. 2022. PMID: 36183136 Free PMC article.
References
-
- Baroja-Fernández E, Muñoz FJ, Akazawa T, Pozueta-Romero J. Reappraisal of the currently prevailing model of starch biosynthesis in photosynthetic tissues: a proposal involving the cytosolic production of ADP-glucose by sucrose synthase and occurrence of cyclic turnover of starch in the chloroplast. Plant and Cell Physiology. 2001;42:1311–1320. - PubMed
-
- Brooks A, Farquhar GD. Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta. 1985;165:397–406. - PubMed
-
- Cruz JA, Emery C, Wüst M, Kramer DM, Lange BM. Metabolic profiling of Calvin cycle intermediates by HPLC-MS using mixed-mode stationary phases. The Plant Journal. 2008;55:1047–1060. - PubMed
-
- Delwiche CF, Sharkey TD. Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant, Cell and Environment. 1993;16:587–591.
-
- Dennis DT, Blakeley SD. Carbohydrate metabolism. In: Buchanan W, Gruissem R, Jones R, editors. The biochemistry and molecular biology of plants. Rockville, MD: American Society of Plant Physiologists; 2000. pp. 630–675.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

