Guanine sulphinate is a major stable product of photochemical oxidation of DNA 6-thioguanine by UVA irradiation
- PMID: 20026585
- PMCID: PMC2847230
- DOI: 10.1093/nar/gkp1165
Guanine sulphinate is a major stable product of photochemical oxidation of DNA 6-thioguanine by UVA irradiation
Abstract
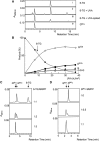
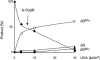
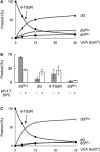
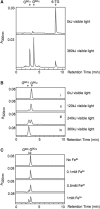
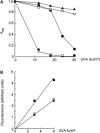
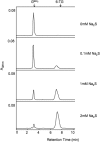
The DNA of patients taking the immunosuppressant and anticancer drugs azathioprine or 6-mercaptopurine contains 6-thioguanine (6-TG). The skin of these patients is selectively sensitive to ultraviolet A radiation (UVA) and they suffer an extremely high incidence of sunlight-induced skin cancer with long-term treatment. DNA 6-TG interacts with UVA to generate reactive oxygen species, which oxidize 6-TG to guanine sulphonate (G(SO3)). We suggested that G(SO3) is formed via the reactive electrophilic intermediates, guanine sulphenate (G(SO)) and guanine sulphinate (G(SO2)). Here, G(SO2) is identified as a significant and stable UVA photoproduct of free 6-TG, its 2'-deoxyribonucleoside, and DNA 6-TG. Mild chemical oxidation converts 6-TG into G(SO2), which can be further oxidized to G(SO3)-a stable product that resists further reaction. In contrast, G(SO2) is converted back to 6-TG under mild conditions. This suggests that cellular antioxidant defences might counteract the UVA-mediated photooxidation of DNA 6-TG at this intermediate step and ameliorate its biological effects. In agreement with this possibility, the antioxidant ascorbate protected DNA 6-TG against UVA oxidation and prevented the formation of G(SO3).
Figures
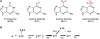
Similar articles
-
Novel DNA lesions generated by the interaction between therapeutic thiopurines and UVA light.DNA Repair (Amst). 2007 Mar 1;6(3):344-54. doi: 10.1016/j.dnarep.2006.11.003. Epub 2006 Dec 22. DNA Repair (Amst). 2007. PMID: 17188583
-
Formation of guanine-6-sulfonate from 6-thioguanine and singlet oxygen: a combined theoretical and experimental study.J Am Chem Soc. 2013 Mar 20;135(11):4509-15. doi: 10.1021/ja400483j. Epub 2013 Mar 8. J Am Chem Soc. 2013. PMID: 23444959
-
Photo-oxidation of 6-thioguanine by UVA: the formation of addition products with low molecular weight thiol compounds.Photochem Photobiol. 2010 Sep-Oct;86(5):1038-45. doi: 10.1111/j.1751-1097.2010.00771.x. Photochem Photobiol. 2010. PMID: 20573042
-
Multiple forms of DNA damage caused by UVA photoactivation of DNA 6-thioguanine.Photochem Photobiol. 2012 Jan-Feb;88(1):5-13. doi: 10.1111/j.1751-1097.2011.01043.x. Epub 2011 Dec 16. Photochem Photobiol. 2012. PMID: 22077233 Review.
-
UVA photosensitization of thiopurines and skin cancer in organ transplant recipients.Photochem Photobiol Sci. 2012 Jan;11(1):62-8. doi: 10.1039/c1pp05194f. Epub 2011 Aug 23. Photochem Photobiol Sci. 2012. PMID: 21860872 Review.
Cited by
-
Photosensitized UVA-Induced Cross-Linking between Human DNA Repair and Replication Proteins and DNA Revealed by Proteomic Analysis.J Proteome Res. 2016 Dec 2;15(12):4612-4623. doi: 10.1021/acs.jproteome.6b00717. Epub 2016 Oct 5. J Proteome Res. 2016. PMID: 27654267 Free PMC article.
-
Formation and repair of oxidatively generated damage in cellular DNA.Free Radic Biol Med. 2017 Jun;107:13-34. doi: 10.1016/j.freeradbiomed.2016.12.049. Epub 2017 Jan 2. Free Radic Biol Med. 2017. PMID: 28057600 Free PMC article. Review.
-
Oxidatively-generated damage to DNA and proteins mediated by photosensitized UVA.Free Radic Biol Med. 2017 Jun;107:101-109. doi: 10.1016/j.freeradbiomed.2016.10.488. Epub 2016 Oct 27. Free Radic Biol Med. 2017. PMID: 27989755 Free PMC article. Review.
-
Excited-State Dynamics of the Thiopurine Prodrug 6-Thioguanine: Can N9-Glycosylation Affect Its Phototoxic Activity?Molecules. 2017 Feb 28;22(3):379. doi: 10.3390/molecules22030379. Molecules. 2017. PMID: 28264514 Free PMC article.
-
DNA damage and repair in human cancer: molecular mechanisms and contribution to therapy-related leukemias.Int J Environ Res Public Health. 2012 Aug;9(8):2636-57. doi: 10.3390/ijerph9082636. Epub 2012 Jul 27. Int J Environ Res Public Health. 2012. PMID: 23066388 Free PMC article. Review.
References
-
- Cadet J, Sage E, Douki T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat. Res. 2005;571:3–17. - PubMed
-
- WHO. International Programme on Chemical Safety, Ultraviolet Radiation. Geneva: World Health Organization; 1994.
-
- Elion GB. The purine path to chemotherapy. Science. 1989;244:41–47. - PubMed
-
- Weinshilboum R. Thiopurine pharmacogenetics: clinical and molecular studies of thiopurine metabolism. Drug Metab. Dispos. 2001;29:601–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

