Farnesoid X receptor, through the binding with steroidogenic factor 1-responsive element, inhibits aromatase expression in tumor Leydig cells
- PMID: 20026603
- PMCID: PMC2820785
- DOI: 10.1074/jbc.M109.052670
Farnesoid X receptor, through the binding with steroidogenic factor 1-responsive element, inhibits aromatase expression in tumor Leydig cells
Abstract
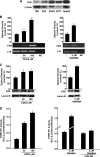
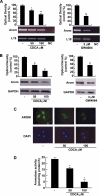
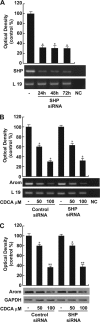
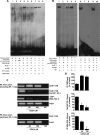
The farnesoid X receptor (FXR) is a member of the nuclear receptor superfamily that regulates bile acid homeostasis. It is expressed in the liver and the gastrointestinal tract, but also in several non-enterohepatic tissues including testis. Recently, FXR was identified as a negative modulator of the androgen-estrogen-converting aromatase enzyme in human breast cancer cells. In the present study we detected the expression of FXR in Leydig normal and tumor cell lines and in rat testes tissue. We found, in rat Leydig tumor cells, R2C, that FXR activation by the primary bile acid chenodeoxycholic acid (CDCA) or a synthetic agonist GW4064, through a SHP-independent mechanism, down-regulates aromatase expression in terms of mRNA, protein levels, and its enzymatic activity. Transient transfection experiments, using vector containing rat aromatase promoter PII, evidenced that CDCA reduces basal aromatase promoter activity. Mutagenesis studies, electrophoretic mobility shift, and chromatin immunoprecipitation analysis reveal that FXR is able to compete with steroidogenic factor 1 in binding to a common sequence present in the aromatase promoter region interfering negatively with its activity. Finally, the FXR-mediated anti-proliferative effects exerted by CDCA on tumor Leydig cells are at least in part due to an inhibition of estrogen-dependent cell growth. In conclusion our findings identify for the first time the activators of FXR as negative modulators of the aromatase enzyme in Leydig tumor cell lines.
Figures




Similar articles
-
Inhibition of Leydig tumor growth by farnesoid X receptor activation: the in vitro and in vivo basis for a novel therapeutic strategy.Int J Cancer. 2013 May 15;132(10):2237-47. doi: 10.1002/ijc.27915. Epub 2012 Nov 7. Int J Cancer. 2013. PMID: 23124354
-
Insulin-like growth factor-I, regulating aromatase expression through steroidogenic factor 1, supports estrogen-dependent tumor Leydig cell proliferation.Cancer Res. 2007 Sep 1;67(17):8368-77. doi: 10.1158/0008-5472.CAN-06-4064. Cancer Res. 2007. PMID: 17804753
-
A steroidogenic factor-1-binding site and cyclic adenosine 3',5'-monophosphate response element-like elements are required for the activity of the rat aromatase promoter in rat Leydig tumor cell lines.Endocrinology. 1998 Dec;139(12):5082-93. doi: 10.1210/endo.139.12.6377. Endocrinology. 1998. PMID: 9832447
-
Regulation of aromatase gene expression in Leydig cells and germ cells.J Steroid Biochem Mol Biol. 2003 Sep;86(3-5):335-43. doi: 10.1016/s0960-0760(03)00343-1. J Steroid Biochem Mol Biol. 2003. PMID: 14623530 Review.
-
Meta-analysis and Consolidation of Farnesoid X Receptor Chromatin Immunoprecipitation Sequencing Data Across Different Species and Conditions.Hepatol Commun. 2021 Oct;5(10):1721-1736. doi: 10.1002/hep4.1749. Epub 2021 Jul 1. Hepatol Commun. 2021. PMID: 34558825 Free PMC article. Review.
Cited by
-
Evidence for Enhanced Exosome Production in Aromatase Inhibitor-Resistant Breast Cancer Cells.Int J Mol Sci. 2020 Aug 14;21(16):5841. doi: 10.3390/ijms21165841. Int J Mol Sci. 2020. PMID: 32823947 Free PMC article.
-
Farnesoid X receptor alpha: a molecular link between bile acids and steroid signaling?Cell Mol Life Sci. 2013 Dec;70(23):4511-26. doi: 10.1007/s00018-013-1387-0. Epub 2013 Jun 20. Cell Mol Life Sci. 2013. PMID: 23784309 Free PMC article. Review.
-
Gender, Estrogen, and Obliterative Lesions in the Lung.Int J Endocrinol. 2017;2017:8475701. doi: 10.1155/2017/8475701. Epub 2017 Apr 2. Int J Endocrinol. 2017. PMID: 28469671 Free PMC article. Review.
-
Estrogen receptor beta binds Sp1 and recruits a corepressor complex to the estrogen receptor alpha gene promoter.Breast Cancer Res Treat. 2012 Jul;134(2):569-81. doi: 10.1007/s10549-012-2090-9. Epub 2012 May 24. Breast Cancer Res Treat. 2012. PMID: 22622808 Free PMC article.
-
Natural Products to Fight Cancer: A Focus on Juglans regia.Toxins (Basel). 2018 Nov 14;10(11):469. doi: 10.3390/toxins10110469. Toxins (Basel). 2018. PMID: 30441778 Free PMC article. Review.
References
-
- Forman B. M., Goode E., Chen J., Oro A. E., Bradley D. J., Perlmann T., Noonan D. J., Burka L. T., McMorris T., Lamph W. W., Evans R. M., Weinberger C. (1995) Cell 81, 687–693 - PubMed
-
- Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., Hull M. V., Lustig K. D., Mangelsdorf D. J., Shan B. (1999) Science 284, 1362–1365 - PubMed
-
- Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., Lehmann J. M. (1999) Science 284, 1365–1368 - PubMed
-
- Kalaany N. Y., Mangelsdorf D. J. (2006) Annu. Rev. Physiol. 68, 159–191 - PubMed
-
- Ananthanarayanan M., Balasubramanian N., Makishima M., Mangelsdorf D. J., Suchy F. J. (2001) J. Biol. Chem. 276, 28857–28865 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

