Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland
- PMID: 20026617
- PMCID: PMC2828142
- DOI: 10.1113/jphysiol.2009.183541
Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland
Abstract
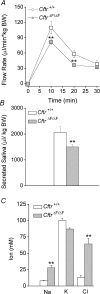
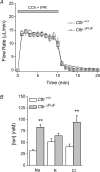
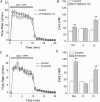
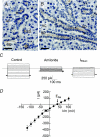
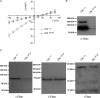
Cystic fibrosis is caused by mutations in CFTR, the cystic fibrosis transmembrane conductance regulator gene. Disruption of CFTR-mediated anion conductance results in defective fluid and electrolyte movement in the epithelial cells of organs such as the pancreas, airways and sweat glands, but the function of CFTR in salivary glands is unclear. Salivary gland acinar cells produce an isotonic, plasma-like fluid, which is subsequently modified by the ducts to produce a hypotonic, NaCl-depleted final saliva. In the present study we investigated whether submandibular salivary glands (SMGs) in F508 mice (Cftr(F/F)) display ion transport defects characteristic of cystic fibrosis in other tissues. Immunolocalization and whole-cell recordings demonstrated that Cftr and the epithelial Na(+) (ENaC) channels are co-expressed in the apical membrane of submandibular duct cells, consistent with the significantly higher saliva [NaCl] observed in vivo in Cftr(F/F) mice. In contrast, Cftr and ENaC channels were not detected in acinar cells, nor was saliva production affected in Cftr(F/F) mice, implying that Cftr contributes little to the fluid secretion process in the mouse SMG. To identify the source of the NaCl absorption defect in Cftr(F/F) mice, saliva was collected from ex vivo perfused SMGs. Cftr(F/F) glands secreted saliva with significantly increased [NaCl]. Moreover, pharmacological inhibition of either Cftr or ENaC in the ex vivo SMGs mimicked the Cftr(F/F) phenotype. In summary, our results demonstrate that NaCl absorption requires and is likely to be mediated by functionally dependent Cftr and ENaC channels localized to the apical membranes of mouse salivary gland duct cells.
Figures

References
-
- Baum M. Developmental changes in proximal tubule NaCl transport. Pediatr Nephrol. 2008;23:185–194. - PubMed
-
- Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol Cell Physiol. 1999;276:C788–795. - PubMed
-
- Berdiev BK, Cormet-Boyaka E, Tousson A, Qadri YJ, Oosterveld-Hut HM, Hong JS, Gonzales PA, Fuller CM, Sorscher EJ, Lukacs GL, Benos DJ. Molecular proximity of cystic fibrosis transmembrane conductance regulator and epithelial sodium channel assessed by fluorescence resonance energy transfer. J Biol Chem. 2007;282:36481–36488. - PubMed
-
- Best JA, Quinton PM. Salivary secretion assay for drug efficacy for cystic fibrosis in mice. Exp Physiol. 2005;90:189–193. - PubMed
-
- Blomfield J, Rush AR, Allars HM, Brown JM. Parotid gland function in children with cystic fibrosis and child control subjects. Pediatr Res. 1976;10:574–578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

