Specific expression of an oxytocin-enhanced cyan fluorescent protein fusion transgene in the rat hypothalamus and posterior pituitary
- PMID: 20026620
- PMCID: PMC2922867
- DOI: 10.1677/JOE-09-0289
Specific expression of an oxytocin-enhanced cyan fluorescent protein fusion transgene in the rat hypothalamus and posterior pituitary
Abstract
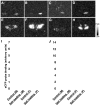
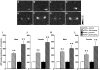
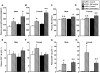
We have generated rats bearing an oxytocin (OXT)-enhanced cyan fluorescent protein (eCFP) fusion transgene designed from a murine construct previously shown to be faithfully expressed in transgenic mice. In situ hybridisation histochemistry revealed that the Oxt-eCfp fusion gene was expressed in the supraoptic nucleus (SON) and the paraventricular nucleus (PVN) in these rats. The fluorescence emanating from eCFP was observed only in the SON, the PVN, the internal layer of the median eminence and the posterior pituitary (PP). In in vitro preparations, freshly dissociated cells from the SON and axon terminals showed clear eCFP fluorescence. Immunohistochemistry for OXT and arginine vasopressin (AVP) revealed that the eCFP fluorescence co-localises with OXT immunofluorescence, but not with AVP immunofluorescence in the SON and the PVN. Although the expression levels of the Oxt-eCfp fusion gene in the SON and the PVN showed a wide range of variations in transgenic rats, eCFP fluorescence was markedly increased in the SON and the PVN, but decreased in the PP after chronic salt loading. The expression of the Oxt gene was significantly increased in the SON and the PVN after chronic salt loading in both non-transgenic and transgenic rats. Compared with wild-type animals, euhydrated and salt-loaded male and female transgenic rats showed no significant differences in plasma osmolality, sodium concentration and OXT and AVP levels, suggesting that the fusion gene expression did not disturb any physiological processes. These results suggest that our new transgenic rats are a valuable new tool to identify OXT-producing neurones and their terminals.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartially of the research reported.
Figures



Similar articles
-
Highly visible expression of an oxytocin-monomeric red fluorescent protein 1 fusion gene in the hypothalamus and posterior pituitary of transgenic rats.Endocrinology. 2011 Jul;152(7):2768-74. doi: 10.1210/en.2011-0006. Epub 2011 May 3. Endocrinology. 2011. PMID: 21540286
-
Differences in acid-induced currents between oxytocin-mRFP1 and vasopressin-eGFP neurons isolated from the supraoptic and paraventricular nuclei of transgenic rats.Neurosci Lett. 2014 Nov 7;583:1-5. doi: 10.1016/j.neulet.2014.09.004. Epub 2014 Sep 8. Neurosci Lett. 2014. PMID: 25220704
-
Exaggerated response of arginine vasopressin-enhanced green fluorescent protein fusion gene to salt loading without disturbance of body fluid homeostasis in rats.J Neuroendocrinol. 2006 Oct;18(10):776-85. doi: 10.1111/j.1365-2826.2006.01476.x. J Neuroendocrinol. 2006. PMID: 16965296
-
Specific expression of optically active reporter gene in arginine vasopressin-secreting neurosecretory cells in the hypothalamic-neurohypophyseal system.J Neuroendocrinol. 2008 Jun;20(6):660-4. doi: 10.1111/j.1365-2826.2008.01706.x. J Neuroendocrinol. 2008. PMID: 18601686 Review.
-
Hypothalamic vasopressin response to stress and various physiological stimuli: visualization in transgenic animal models.Horm Behav. 2011 Feb;59(2):221-6. doi: 10.1016/j.yhbeh.2010.12.007. Epub 2010 Dec 23. Horm Behav. 2011. PMID: 21185297 Review.
Cited by
-
Using transgenic mouse models to study oxytocin's role in the facilitation of species propagation.Brain Res. 2010 Dec 10;1364:216-24. doi: 10.1016/j.brainres.2010.08.042. Epub 2010 Aug 20. Brain Res. 2010. PMID: 20732312 Free PMC article. Review.
-
Modulation/physiology of calcium channel sub-types in neurosecretory terminals.Cell Calcium. 2012 Mar-Apr;51(3-4):284-92. doi: 10.1016/j.ceca.2012.01.008. Epub 2012 Feb 17. Cell Calcium. 2012. PMID: 22341671 Free PMC article. Review.
-
Fluorescent visualization of oxytocin in the hypothalamo-neurohypophysial system.Front Neurosci. 2014 Jul 23;8:213. doi: 10.3389/fnins.2014.00213. eCollection 2014. Front Neurosci. 2014. PMID: 25100939 Free PMC article. Review.
-
Vasopressin and oxytocin in sensory neurones: expression, exocytotic release and regulation by lactation.Sci Rep. 2018 Aug 30;8(1):13084. doi: 10.1038/s41598-018-31361-1. Sci Rep. 2018. PMID: 30166555 Free PMC article.
-
REVIEW: Oxytocin: Crossing the bridge between basic science and pharmacotherapy.CNS Neurosci Ther. 2010 Oct;16(5):e138-56. doi: 10.1111/j.1755-5949.2010.00185.x. Epub 2010 Jul 7. CNS Neurosci Ther. 2010. PMID: 20626426 Free PMC article. Review.
References
-
- Abbott A. The Renaissance rat. Nature. 2004;428:464–466. - PubMed
-
- Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiological Reviews. 2001;81:1197–1267. - PubMed
-
- Cozzi J, Fraichard A, Thiam K. Use of genetically modified rat models for translational medicine. Drug Discovery Today. 2008;13:488–494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

