Autophosphorylation at serine 1981 stabilizes ATM at DNA damage sites
- PMID: 20026654
- PMCID: PMC2806275
- DOI: 10.1083/jcb.200906064
Autophosphorylation at serine 1981 stabilizes ATM at DNA damage sites
Erratum in
- J Cell Biol. 2010 Feb 8;188(3):443
Abstract
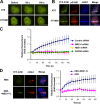
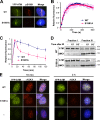
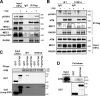
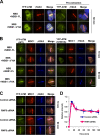
Ataxia telangiectasia mutated (ATM) plays a critical role in the cellular response to DNA damage. In response to DNA double-strand breaks (DSBs), ATM is autophosphorylated at serine 1981. Although this autophosphorylation is widely considered a sign of ATM activation, it is still not clear if autophosphorylation is required for ATM functions including localization to DSBs and activation of ATM kinase activity. In this study, we show that localization of ATM to DSBs is differentially regulated with the initial localization requiring the MRE11-RAD50-NBS1 complex and sustained retention requiring autophosphorylation of ATM at serine 1981. Autophosphorylated ATM interacts with MDC1 and the latter is required for the prolonged association of ATM to DSBs. Ablation of ATM autophosphorylation or knock-down of MDC1 protein affects the ability of ATM to phosphorylate downstream substrates and confer radioresistance. Together, these data suggest that autophosphorylation at serine 1981 stabilizes ATM at the sites of DSBs, and this is required for a proper DNA damage response.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

